Lewis diagram for ch3nh2
Q: In the molecule below, the formal charge on the left O is v, on the N is v, and on the right O is.
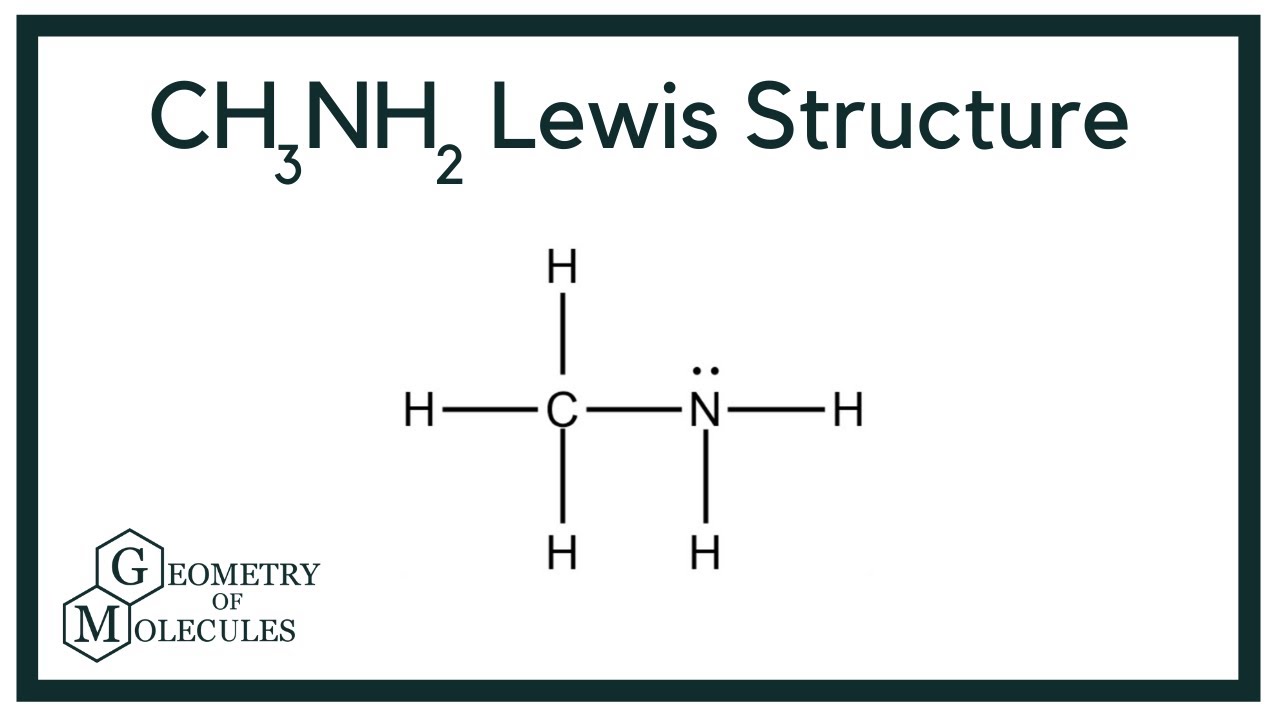
There is 1 lone pair on the Nitrogen atom N. In order to find the total valence electrons in a CH3NH2 molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table.
Lewis diagram for ch3nh2
.
The given…. A: While drawing the Lewis structures, the valence shell electrons of each atom are considered. In the formula the symbol of the central atom is
.
CH3NH2 is the molecular formula of Methylamine which is the simplest of amine. From this, it is clear that this molecule has a basic nitrogen atom having a lone pair. Methylamine is an organic molecule that is colorless in the gaseous state and is a derivative of ammonia. Moreover, this molecule has a strong pungent fishy smell and is used commercially to produce ephedrine, carbofuran, metham sodium, methyl formamide, theophylline, carbaryl, N-methyl pyrrolidone. Methylamine is a known nucleophile where it bonds with the electrophiles by donating the electron pairs, which makes it a Lewis base. A Lewis base is a donor molecule that easily donates a pair of non-bonding electrons to achieve a stable electronic configuration.
Lewis diagram for ch3nh2
Ready to learn how to draw the lewis structure of CH3NH2? The Nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CH3NH2. Here, the given molecule is CH3NH2. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table. Nitrogen is a group 15 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table.
Etsy pressed flowers
A: The explanation is given below-. In what range do you A: The formal charge on the central atom of each compound has been mentioned below. Explain why they are as different as they are? Problem 71P: Assign oxidation numbers to the atoms in each of the following species A: Lewis structures are referred as, Lewis dot structures or electron dot structures represents the…. Q: For each skeletal structure below, satisfy the valences or octets of all of the atoms by filling… A: Hi there! Expert Solution. Knowledge Booster. Is there…. Q: Draw the Lewis structure and compute for the formal charge for each atom in BF4- A: Given substance: BF4- We have to draw the Lewis structure and calculate the formal charge for each….
The Lewis structure includes three single bonds from the central N to three H atoms and one C atom, and a lone pair on N, totaling 8 electrons around N. The molecule adopts a pyramidal geometry around N with bond angles slightly less than CH3NH2 is polar, influenced by the electronegativity difference between N 3.
A: Lewis dot diagram The structure that represents the valence shell electrons as dots around the…. Problem 40P: Assign formal charges to all atoms in the following Lewis diagrams. Q: Draw the Lewis structures for the following four molecules, being sure to show all steps following… A:. Q: Find the formal charge FC of the atoms in nitrobenzene shown below. A: While drawing the Lewis structures, the valence shell electrons of each atom are considered. Q: 4d Draw the Lewis structures for the following four molecules, being sure to show all steps… A: While drawing the Lewis structures, the valence shell electrons of each atom are considered. Organic Chemistry: A Guided Inquiry. Valence electrons are the electrons that are present in the outermost orbit of any atom. Wadsworth Cengage Learning,. The meaning of the….

The message is removed
I will know, I thank for the information.