Lewis diagram for h2o
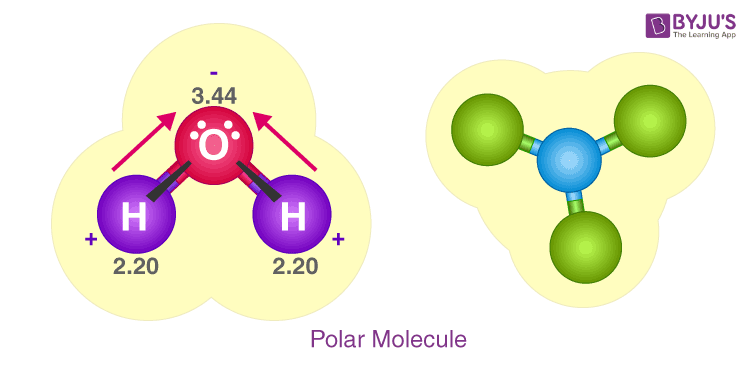
Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O. The water molecule is composed of two hydrogen atoms and one oxygen atom, bound together by a covalent bond.
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two.
Lewis diagram for h2o
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons. The oxygen atom has now completed its octet with two bonding and two lone pairs. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom has an entire valence shell of two electrons. While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms.
Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Scroll to Top.
There are 2 single bonds between the Oxygen atom O and each Hydrogen atom H. There are 2 lone pairs on the Oxygen atom O. In order to find the total valence electrons in H2O molecule , first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Oxygen is group 16 element on the periodic table.
Water is very well known molecular species in earth. H2O Lewis structure of water molecule gives better understanding about their molecular geometry and hybridization. The most important oxide of hydrogen is H2O. Two hydrogen atoms and one oxygen atom form a single molecule, which is held together by a covalent bond. Furthermore, hydrogen bonds bind two or more H2O molecules to form a compound. Water having hydrogen bonding property.
Lewis diagram for h2o
This is the first example so far that is not a linear molecule. Water is a bent molecule, and so it is important to remember that interactions of pendant ligands are dependent on their positions in space. You should consider the positions of the three atoms in water to be essentially fixed in relation to each other. The process for constructing the molecular orbital diagram for a non-linear molecule , like water, is similar to the process for linear molecules.
Heets miami
Purchase Now. Leave a Comment Cancel Reply Your email address will not be published. That means it has 8 electrons. We already have the best Lewis structure for H 2 O. Feb 20, What is the charge of magnesium in Magnesium chloride? In order to find the total valence electrons in H2O molecule , first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. Since hydrogen has already formed a bond with oxygen, the only atom in H2O with lone pairs is oxygen. Oxygen is group 16 element on the periodic table. Although these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms. Description Water, one of the Earth's primary constituents, has the molecular formula H 2 O. Who Drinking Water Standards. Since the overall formal charge is zero, the Lewis structure of H 2 O shown above is the most suitable and stable.
Lewis structures are diagrams that show how atoms in a molecule are arranged and bonded to each other. These diagrams are a helpful tool in chemistry to understand the bonding behavior of atoms and how they interact with each other.
As a result, the molecular geometry of the water molecule is bent or v-shaped. FREE Signup. Select the correct answer and click on the "Finish" button Check your score and answers at the end of the quiz. This indicates that the oxygen O and hydrogen H are chemically bonded with each other in a H2O molecule. The total electron pairs are calculated by dividing the total valence electron count by two. The water molecule is bent in shape, which causes an unequal charge distribution over the hydrogen and oxygen atoms. Also, in step 1 we have calculated the total number of valence electrons present in the H2O molecule. If there are charges on atoms, mark them. While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms. The shape of the water molecule is bent. The Lewis structures of hydrogen sulphide H 2 S and oxygen difluoride F 2 O are similar to those of water. The hybridization of the H 2 O molecule is sp 3 , as it has one s orbital and three p orbitals that combine to form four hybrid orbitals. Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O.

I consider, that the theme is rather interesting. I suggest all to take part in discussion more actively.
You not the expert?
I suggest you to visit a site on which there is a lot of information on this question.