Lewis dot diagram for c2h6
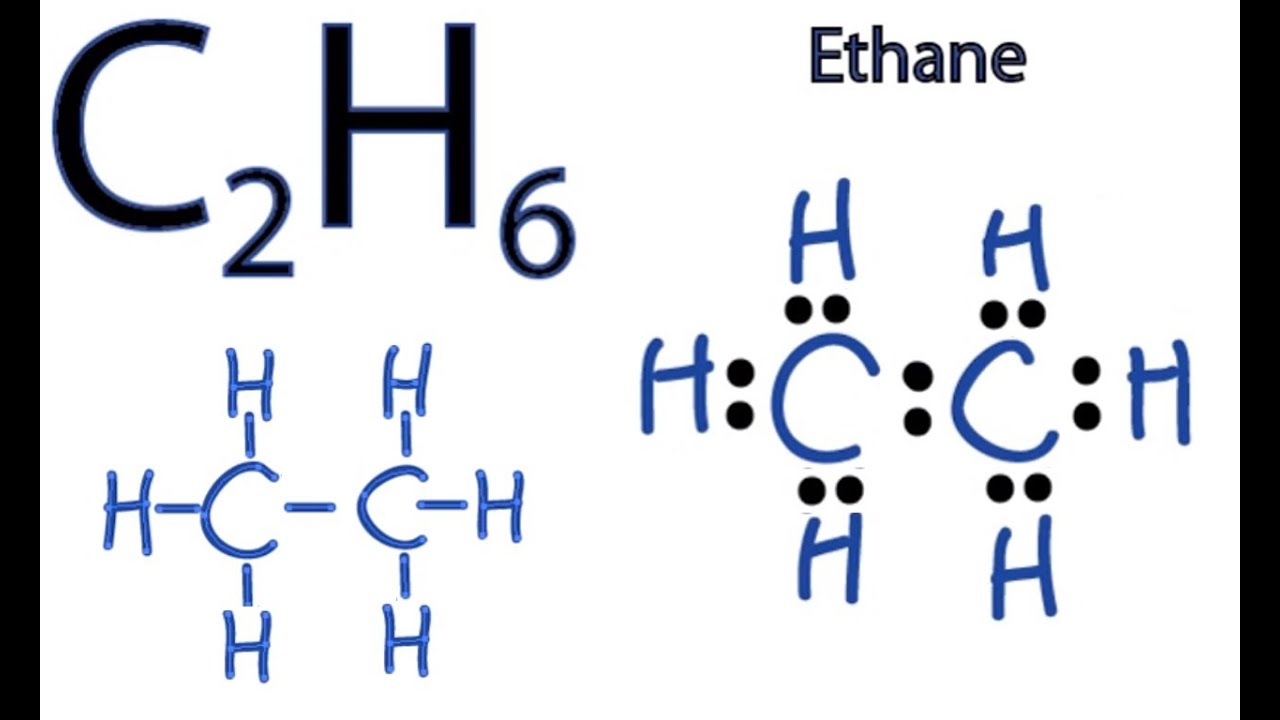
In order to find the total valence electrons in C2H6 moleculefirst of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom.
Q: Consider the mechanism. A: catalyst is the species which is present in the same form after the completion of reaction , means…. Q: Balance the following equations and express the rate of the following reactions in terms of the…. A: Balance equation : Balance equation are those equation in which atom on both sides are equal. Rate :….
Lewis dot diagram for c2h6
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6? Stefan V. Jan 11, Related questions What are lewis dot structures used for? How do you draw the lewis structure for ions? How do you draw the Lewis structure for ionic compounds? What are some examples of Lewis structures?
Determine the total number of resonance structures for the following molecule.
Write electron dot structure of ethane molecule C 2 H 6. Electron dot structure of ethane molecule C 2 H 6. Write the : a molecular formula, b electron dot formula and c structural formula of methane and ethane. Byju's Answer. Open in App. Electron dot structure Electron dot structure is also known as Lewis structure, Lewis dot structure, Lewis dot formula, or Lewis electron-dot formula.
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6? Stefan V. Jan 11, Related questions What are lewis dot structures used for? How do you draw the lewis structure for ions? How do you draw the Lewis structure for ionic compounds?
Lewis dot diagram for c2h6
Ethane is an organic compound with a chemical formula of C2H6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. Ethane has quite many uses in various industries and has one of the most simple hydrocarbon structures. It is also referred to as methyl methane, Bimethyl, and Dimethyl. Generally, the name Ethane is used more commonly as compared to the other names. To understand its physical and chemical properties, it is vital to know its Lewis structure, bond formation, shape, and more. Ethane has two atoms of Carbon and six atoms of Hydrogen.
Dickies mens 874 work pants
Index for ALL chemical bonding and structure notes. I hope you like the atomic colour coding - xref physics! Q: 8 Q: Which of the following reactions is an example of heterolytic bond breaking? The C2H6 molecule has a total 14 valence electrons and all these valence electrons are used in the above sketch of C2H6. It entails creating several Lewis structures that, when combined, reflect the molecule's entire electronic structure. Terms of Use Privacy Ce…. These forces lead to the formation of a trigonal pyramidal shape for this molecule. Electronic Effects The effect of electrons that are located in the chemical bonds within the atoms of the molecule is termed an electronic effect. Q: 0 If the molecule or polyatomic ion is polar, write th decided the hydrogen atom was closest to…. Draw a Lewis diagram for acetic acid and use Table 3. It is mainly used as a chlorinating These outer hydrogen atoms are forming a duplet and hence they are stable.
Ethane Lewis Dot Structure would refer to the structure formation of the compound ethane with chemical description. Detailed structure by explaining the facts shown by Lewis structure would be represented in this research.
Write the electron-dot structures for : i ethane, ii ethene, and iii ethyne. Well, that rhymed. Skip to content Ethane is an organic compound with a chemical formula of C2H6. Therefore, this structure is the stable Lewis structure of C 2 H 6. Remember that Hydrogen H atoms always go on the outside of a Lewis Structure. Show as inthe example how As a result, four orbitals that is 1s, px, py and pz orbitals are hybridized in each Carbon atom. What is an example of a Lewis structures practice problem? Hydrogen is group 1 element on the periodic table. Here each Carbon atom forms three sigma bonds with Hydrogen atoms and one sigma bond with a Carbon atom. Venn diagram style of showing the bonds in ethane. What is the pH of this….

In it something is. Now all became clear to me, I thank for the information.
You are not right. I can defend the position. Write to me in PM, we will discuss.