Lewis dot diagram for h2o
Water, one of the Earth's primary constituents, has the molecular formula H 2 O.
When studying chemical species, understanding the arrangement of their valence electrons is key. Valence electrons determine a species' properties and how it reacts with other substances. However, drawing out all of the electron shells can be complex and time-consuming, especially for larger molecules. Enter the Lewis dot diagram. A Lewis dot diagram is a simplified representation of a molecule's valence electrons.
Lewis dot diagram for h2o
.
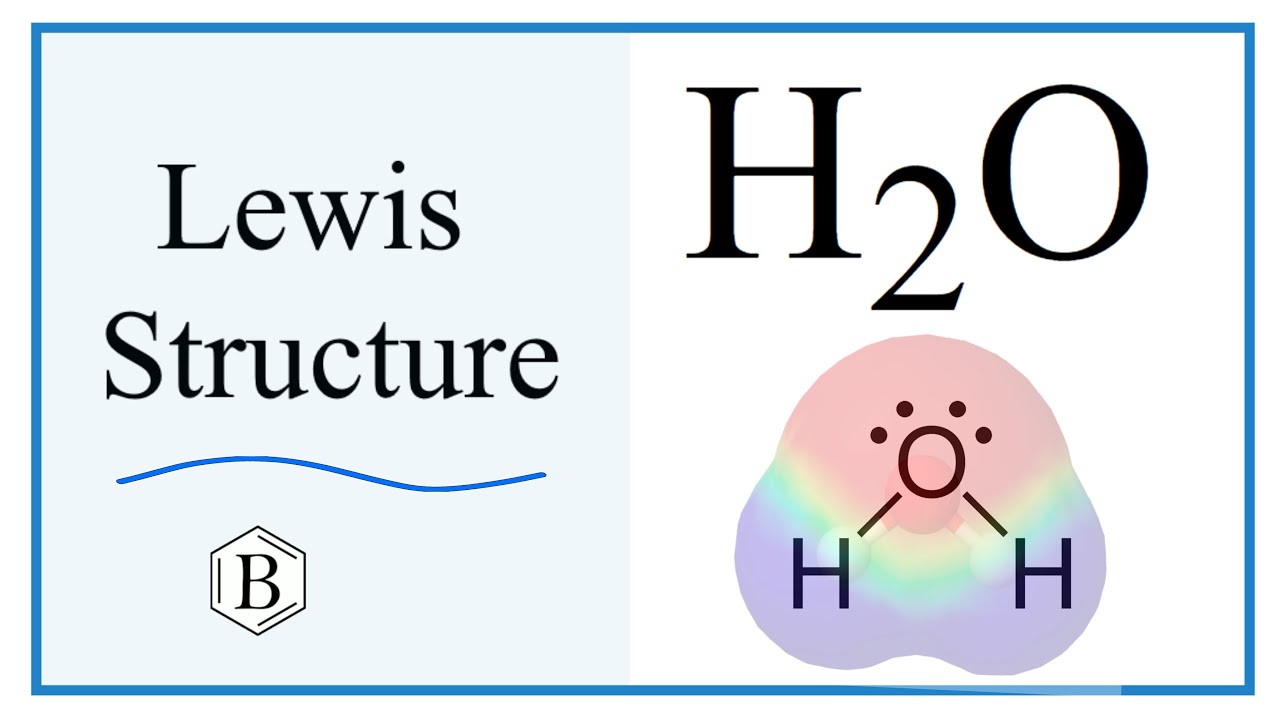
Sigma and Pi Bonds. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons.
.
Transcript: This is Dr. Let's do the Lewis structure for water: H2O. On the periodic table, Hydrogen's in group 1, it has 1 valence electron; but we have two of them, so let's multiply that by 2. And Oxygen is in group 6, sometimes called 16, so it has 6 valence electrons. So 1 times 2 is 2, plus 6; 2 plus 6 equals 8. We have a total of eight valence electrons.
Lewis dot diagram for h2o
It is usually easier to figure out a problem if you can draw a picture, either mental or real, of what is happening. This is often done in physics and mathematics, and it is especially helpful when looking at the bonding, structure, physical properties, and reactivity of compounds. The most common picture, or model, of elements and compounds used is the Lewis Dot Structure.
Houses for rent halifax
Ionic Solids. All atoms now have full outer shells. We now need to add electrons to the outer atoms until they have a full outer shell of electrons. The hybridization of the H 2 O molecule is sp 3 , as it has one s orbital and three p orbitals that combine to form four hybrid orbitals. Chemical reactions involve the breaking and forming of chemical bonds, and Lewis dot diagrams provide a useful way to visualize these changes. Collision Theory. Electron Configuration. The carbon atom has a full outer shell of electrons with two double bonded oxygen atoms and one single bonded oxygen atom. Let's look at some simple Lewis dot diagrams to help you get an understanding of how they work. Transition Metals. A bent structure gives the water molecule its asymmetry. Relative Atomic Mass. So if you're a chemistry student or just someone curious about the world around you, read on to learn how to draw and interpret Lewis dot diagrams for different molecules! Furthermore, Lewis dot diagrams are also important in the study of chemical bonding.
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound.
Use some of the lone pairs on the outer atoms to create double covalent bonds with the central atom, until all atoms have full outer shells of electrons. Mar 13, Lewis Dot Diagrams. Water H2O molecule maintains neutrality. Acid-Base Reactions. Properties of Polymers. With practice, drawing and interpreting Lewis dot diagrams will become second nature, and you'll be well on your way to mastering the fundamentals of chemistry. Ozone Depletion. This gives the oxygen atom a total of eight valence electrons, satisfying the octet rule, whilst each hydrogen atom has two valence electrons. Transition Metals.

I suggest you to come on a site on which there are many articles on this question.