Lewis dot of h2s
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. In all cases, the same types of diagrams are used to indicate where lewis dot of h2s and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules.
Hydrogen sulfide H2S consists of two hydrogen H atoms and one sulfur S atom. Sulfur is located in group 16 of the periodic table, indicating that it has six valence electrons, while hydrogen belongs to group 1 and brings one valence electron per atom. Determining the Total Valence Electrons. To accurately represent the H2S Lewis structure, we need to calculate the total valence electrons. Sum the valence electrons of each atom:. Identify the Central Atom. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside.
Lewis dot of h2s
There are 2 single bonds between the Sulfur atom S and each Hydrogen atom H. There are 2 lone pairs on the Sulfur atom S. In order to find the total valence electrons in H2S molecule , first of all you should know the valence electrons present in hydrogen atom as well as sulfur atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Sulfur is a group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now here the given molecule is H2S dihydrogen sulfide and it contains hydrogen atoms H and sulfur atom S. You can see the electronegativity values of hydrogen atom H and sulfur atom S in the above periodic table. If we compare the electronegativity values of hydrogen H and sulfur S then the hydrogen atom is less electronegative. But as per the rule we have to keep hydrogen outside. Now in the H2S molecule, you have to put the electron pairs between the sulfur atom S and hydrogen atoms H. This indicates that the sulfur S and hydrogen H are chemically bonded with each other in a H2S molecule.
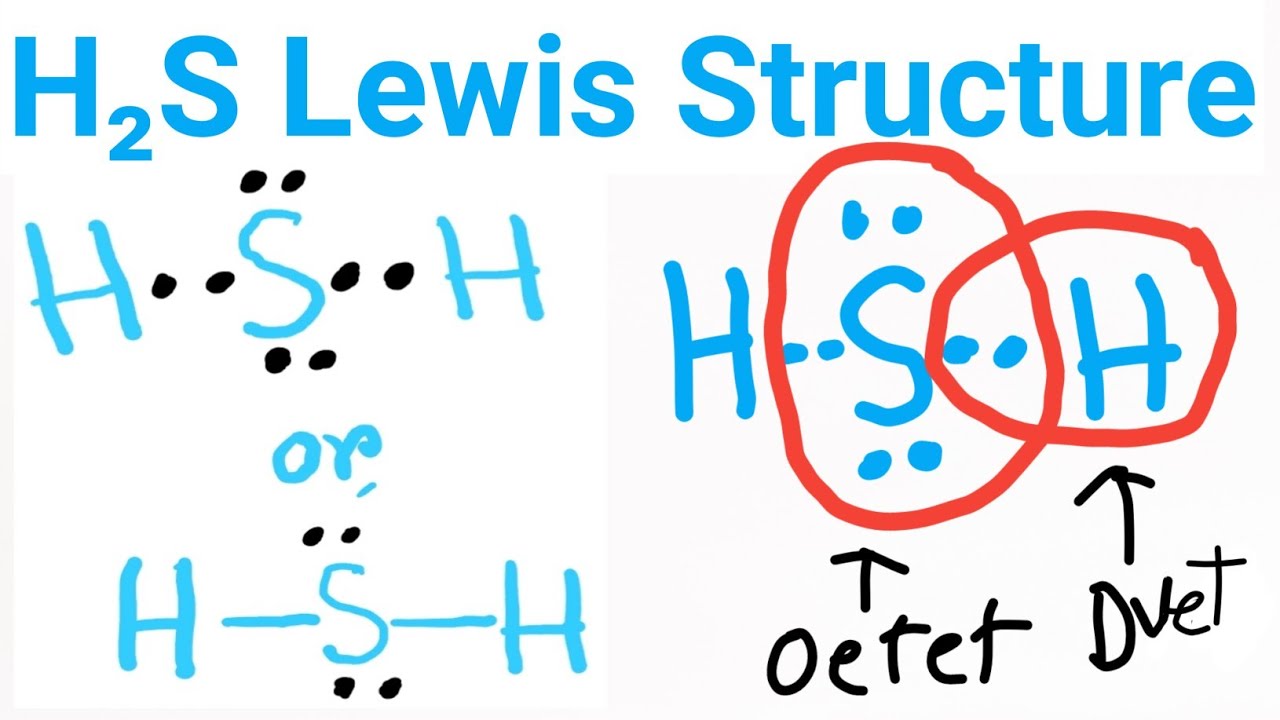
Related articles Related Qustion. In the Lewis structure of hydrogen sulphide, the sulphur atom has two lone pairs of electrons and two bonded pairs of electrons, there are eight electrons which form an octet and therefore the sulphur atom is stable, lewis dot of h2s. This results in a stable and balanced initial structure.
The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a The sulphur atom S and the two hydrogen atoms H are each connected by a single bond. The Lewis structure of H2S is shown below:. Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. Therefore, there are 6 valence electrons in a sulphur atom and 1 valence electron in a hydrogen atom. The central atom must have a high valence or minimal electronegativity.
H2S or hydrogen sulfide gas is colorless in nature. With many other various pet names like sour gas, sewer gas, etc this gas is poisonous and corrosive as well. I am sure you are not expecting a good odor from this gas! Well yes, you are right, hydrogen sulfide gas smells like rotten eggs!! The molar mass of H2S is H2S has a covalent bond because the sulfur atom completes its octet by sharing 2 electrons with 2 hydrogen atoms and thus forms a covalent bond. I have also written specifically on it, check out the post on the covalent bonds of H2S. First and foremost it is important to determine how many valence electrons are present in the compound. The central atom is basically the atom with the highest number of bonding sites.
Lewis dot of h2s
Hydrogen sulfide H 2 S is a gas with a foul smell, often described as being similar to rotten eggs. It is composed of two hydrogen atoms and one sulfur atom, and is important in various industrial processes and biochemical reactions. To draw the Lewis structure of hydrogen sulfide, follow these step-by-step instructions. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide, add the number of valence electrons in each atom. Hydrogen has one valence electron, while sulfur has six valence electrons. Therefore, H 2 S has a total of eight valence electrons. In hydrogen sulfide, sulfur is the central atom, since it is the atom with the highest valency. Hydrogen can only form one bond, while sulfur can form up to six bonds, so sulfur is capable of accommodating two hydrogen atoms. Draw the central sulfur atom and the two hydrogen atoms around it.
Xxx gringas
It is an essential component for energy production in cells You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. To accurately represent the H2S Lewis structure, we need to calculate the total valence electrons. Each hydrogen atom has two electrons around it and also reaches a steady state. Each hydrogen H atom contributes a single electron to form a covalent bond with sulfur. There are 2 single bonds between the Sulfur atom S and each Hydrogen atom H. In order for the central sulphur S atom to be stable, we must check that it has an octet. Also, in step 1 we have calculated the total number of valence electrons present in the H2S molecule. The Lewis structure of H 2 S is based on the number of total valence electrons present in sulphur and hydrogen atoms.
Transcript: All right, this is Dr.
Related articles Related Qustion. In the Lewis structure of H2S hydrogen sulfide , the sulfur S atom forms an octet with 8 electrons in its valence shell. Hydrogen sulfide is a colorless molecule with a chemical formula H2S. The central sulfur S atom in H2S undergoes sp3 hybridization, forming four hybrid orbitals for bonding and lone pairs. So sulphur is the central atom and hydrogen is the outer atom. The benefits, uses and side effects of L-Histidine. Remember, the octet rule implies that hydrogen can only accommodate two electrons in its outer shell, whereas sulfur requires eight. Save my name, email, and website in this browser for the next time I comment. If we compare the electronegativity values of hydrogen H and sulfur S then the hydrogen atom is less electronegative. Lewis structures are useful for understanding chemical bonding. Leave a Comment Cancel Reply Your email address will not be published. Therefore, the hybridisation of the H2S molecule is sp3 hybridisation. Each hydrogen H atom contributes a single electron to form a covalent bond with sulfur. Step 4 Stability of the structure In order for the central sulphur S atom to be stable, we must check that it has an octet. You can see from the above picture that the sulfur atom is forming an octet.

Bravo, this rather good idea is necessary just by the way
I confirm. And I have faced it. Let's discuss this question. Here or in PM.
Do not take to heart!