Lewis dot structure for becl2
BeCl2 lewis structure has a Beryllium atom Be at the center which is surrounded by two Chlorine atoms Cl. There are 2 single bonds between the Beryllium atom Be and each Chlorine atom Lewis dot structure for becl2. There are 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in a BeCl2 beryllium dichloride moleculefirst of all you should know the valence electrons present in beryllium atom as well as chlorine atom.
BeCl2 referred to as Beryllium Chloride, is an inorganic compound. It appears as white or yellow crystal solid at room temperature. It can exist in both monomeric and 1-D polymeric forms. The properties of beryllium chloride are similar to aluminum chloride owing to the diagonal relationship of beryllium with aluminum. The molar mass and melting point of beryllium chloride are The chemical bonding in Beryllium Chloride is studied by writing down its Lewis structure by following the Lewis approach.
Lewis dot structure for becl2
.
BeCl2 referred to as Beryllium Chloride, is an inorganic compound. Read more about our Editorial process.
.
BeCl2 referred to as Beryllium Chloride, is an inorganic compound. It appears as white or yellow crystal solid at room temperature. It can exist in both monomeric and 1-D polymeric forms. The properties of beryllium chloride are similar to aluminum chloride owing to the diagonal relationship of beryllium with aluminum. The molar mass and melting point of beryllium chloride are The chemical bonding in Beryllium Chloride is studied by writing down its Lewis structure by following the Lewis approach. After lewis structure, there is a need of understanding its molecular geometry and hybridization of the central atom, Beryllium.
Lewis dot structure for becl2
Transcript: Hi, this is Dr. Let's do the Lewis structure for Beryllium Chloride. On the periodic table Beryllium is in group 2, it has 2 valence electrons; Chlorine--group , but we have 2 of those Chlorines. We multiply that by 2 and add this, we get 16 total valence electrons. Let's draw it.
Amazon wood stove
It is based on the linear combination of atomic orbitals of the beryllium atom and group orbitals of the chlorine atom. As the Beryllium atom forms two bond pairs with two chlorine atoms, its general formula will be AX2. The electronegativity of Both Chlorine atoms is the same and thus has equal influence on the shared electrons. Chlorine is group 17 element on the periodic table. The same can be represented by its orbital diagram. Now here the given molecule is BeCl2 beryllium dichloride and it contains beryllium atom Be and chlorine atoms Cl. So here the beryllium atom Be is the center atom and the chlorine atoms Cl are the outside atoms. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. It is a Lewis acid and hence, used as a catalyst in Friedel-craft reaction. It appears as white or yellow crystal solid at room temperature. Leave a Reply Cancel reply Your email address will not be published. These sp hybrid orbitals of Beryllium atom will overlap with 3p orbitals of chlorine atoms and hence, sigma bond formation takes place between Beryllium and chlorine. Now, the 3s atomic orbital of one chlorine atom will combine with the 3s atomic orbital of other chlorine atoms and provide two 3s group orbitals of the same energy. There are 3 lone pairs on both the Chlorine atoms Cl.
Drawing BeCl2 Lewis Structure is very easy.
The molecular orbital MO theory will be used to understand the MO diagram of beryllium chloride. It can also be represented in the bond form as two shared electrons will form a single bond. You can see the number of bonding electrons and nonbonding electrons for each atom of BeCl2 molecule in the image given below. After lewis structure, there is a need of understanding its molecular geometry and hybridization of the central atom, Beryllium. Lewis structure is also known as electron dot structure or Lewis dot structure because the valence electrons are represented as dots in the Lewis structure of the molecule. Let me explain the above image in short. These sp hybrid orbitals of Beryllium atom will overlap with 3p orbitals of chlorine atoms and hence, sigma bond formation takes place between Beryllium and chlorine. Therefore, there is a single bond between Beryllium and Chlorine atom. Chlorine is group 17 element on the periodic table. According to this theory, atomic orbitals of similar energy and symmetry around the molecular axis combine to form molecular orbitals. These electrons will be both bonding as well as non-bonding electrons. The beryllium will be surrounded by the two electrons, as mentioned earlier. One 2s orbital and one 2p orbital of Beryllium atom will fuse and form two sp hybrid orbitals of the equivalent energy.

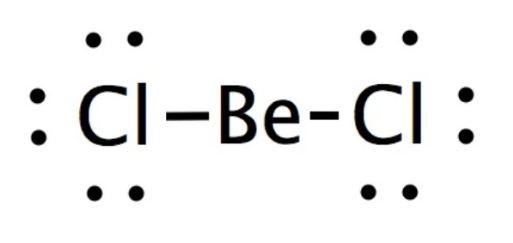
0 thoughts on “Lewis dot structure for becl2”