Lewis dot structure for o3
The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level.
This article in whole includes the details on the topic and a short note on the resonance structure of O3. This article also includes the topics like bond length and major and minor contributors of resonance. Resonance structures are a more accurate representation of a Lewis dot structure than Lewis dot structures because they clearly illustrate the bonding between molecules. Not all resonance structures are created equal; some are superior to others. The better ones have the fewest formal charges, the most electronegative atoms have the most formal charges, and the structure maximizes bonding.
Lewis dot structure for o3
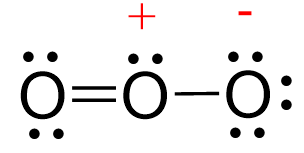
Because, around the central oxygen, there are 5 electrons 2 from the double bond, 1 from the single bond, and 2 from the lone pair , we assign this centre a positive charge, and of course we can assign each terminal oxygen a negative charge alternately by resonance. What do we find experimentally? Thus, by simply knowing how to draw a Lewis structure, counting the electrons, and using VSEPR , we have predicted the structure of a gaseous molecule, which we can't see, but we can smell. I think that is pretty clever given the short! Why is the Lewis structure of ozone important? Jun 6, Because it's a simple predictor of molecular shape. Related questions How is the Lewis structure of an ion written? Are non-valence electrons represented in a Lewis dot diagram? How is the total number of electrons represented in a Lewis structure determined? What are lone pairs and how are they represented in a Lewis dot diagram? Is it possible to draw Lewis dot diagrams for ionic compounds? How can I draw a Lewis dot diagram for carbon dioxide? Can Lewis structures predict the shape of a molecule? What is the electron dot diagram for carbon?
Learn more topics related to Chemistry. Molecules containing three electron pairs have a trigonal planar domain shape. Notify me of followup comments via e-mail.
.
Due to vast global warming and the rapid increase of temperature on earth, the ozone layer of the stratosphere has a hole in it. This causes severe climate change and environmental damage. A pale blue gas with a molar mass of Formed from the dioxide molecule, this molecule having three oxygen atoms is very crucial from the chemistry point of view. If you want to dive into his molecule, let us fasten our seat belts! Because I am going to make you travel through all the essential concepts and explanations related to bonding within ozone. To be very precise, Lewis Structure is the name given to the structural representation of a molecule. It is the diagrammatic layout for understanding the nitty-gritty of chemical bonding. A very essential concept of molecular chemistry, the following steps dictate how you can successfully draw Lewis Structure:.
Lewis dot structure for o3
The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na — 1s 2 2s 2 2p 6 3s 1 , Cl — 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:. For example, iron has eight valence electrons: Fe — 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6.
Born january 2
Why is the ozone angle greater than the water angle? Contributors, both major and minor One of the contributing structures may bear a greater resemblance to the actual molecule than another in the sense of energy and stability. Major contributors are the most stable contributing structures. Impact of this question views around the world. Related articles. That is why, in comparison to the water molecule, the ozone molecule has a greater bond angle, i. Ozone has one double bond and one single bond in its Lewis structure. Click here to get more info on the aforementioned topic. Notice also that the molecular geometry of ozone is bent because the central atom is connected to two atoms and has one lone pair. Table of Content. Related questions How is the Lewis structure of an ion written? This lesson walks you through each step of drawing the Lewis structure of O 3. For example: Na — 1s 2 2s 2 2p 6 3s 1 , Cl — 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:. Why does ozone have a bent shape?
Ozone is one of the most common examples used to study the Lewis structure. The molecule of Ozone has three oxygen atoms. It is written as O3 in the core chemistry equations.
Can Lewis structures predict the shape of a molecule? Energetically unfavorable and thus less advantageous structures play a limited role. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. Use a pair of electrons to form a bond between each pair of bound atoms. In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. We now have 14 valence electrons left, and since the middle oxygen has two bonds while the terminal oxygens have one, we add them to terminal oxygens as two pairs: Next, check if the atoms have octet. Contributors, both major and minor One of the contributing structures may bear a greater resemblance to the actual molecule than another in the sense of energy and stability. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application. Why is the ozone angle greater than the water angle? Keep in mind that the actual structure of the molecule is intermediate between the two or more resonance structures and is called a resonance hybrid. This is identical to what occurs in the water molecule, with the exception that water has two lone pairs of electrons surrounding the central oxygen atom. Are non-valence electrons represented in a Lewis dot diagram? Trending Topics.

0 thoughts on “Lewis dot structure for o3”