Lewis structure for ch3br
It consists of one carbon atom, three hydrogen atoms, and one bromine atom. This compound is commonly used as a fumigant and pesticide and is highly toxic to humans and animals. The CH3Br Lewis structure and its geometry help to understand the bonding, lewis structure for ch3br, reactivity, and properties of the molecule. The CH3Br Lewis structure refers to a visual representation of the arrangement of atoms and electrons in the molecule.
Bromomethane is an organobromine compound usually produced synthetically. However, it is also known to occur in oceans in a small amount. It occurs as a non-flammable, colorless, and odorless gas. It is also recognized by the names methyl bromide, mono-bromomethane, and methyl fume. Trade names of Bromomethane are Embafume and Terabol. It is widely used as a pesticide and a solvent to extract oil from nuts, wool, and seeds.
Lewis structure for ch3br
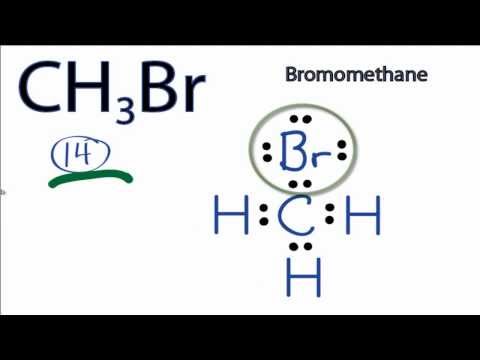
Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound. It has only one carbon atom. Carbon atom is the center atom and bromine atom has 3 lone pairs. We will learn how to draw lewis structure of CH 3 Br step by step in this tutorial. Figure of CH 3 Br lewis structure is given above and you can see how atoms are joint with other atoms. Also, there are no charges on atoms and CH 3 Br also does not have an overall charge. An organobromine compound which exist as a nonflammable gas at room temperature. It is an ozone-depleting compound with one bromine atom. Methyl bromide is an acute toxic chemical and should carefully handle with proper safety equipments. There are several steps to draw the lewis structure of CH 3 Br. Each step is explained in detail in this tutorial.
Trade names of Bromomethane are Embafume and Terabol.
.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Brom-methan [German]. Bromometano [Italian]. Bromure de methyle [French]. Bromuro di metile [Italian]. Broommethaan [Dutch]. Methylbromid [German]. Metylu bromek [Polish].
Lewis structure for ch3br
The bromomethane chemical formula is CH3Br. The carbon, bromine, and hydrogen elements come as the member of the carbon, halogen, and hydrogen family groups from the periodic table respectively. The valence electrons in carbon, bromine, and hydrogen are four, seven, and one respectively. Bromomethane is used as an organic volatile solvent in organic reactions. A three-step approach for drawing the CH3Br Lewis structure can be used. The first step is to sketch the Lewis structure of the CH3Br molecule, to add valence electron around the carbon atom; the second step is to add valence electrons to the one bromine and three hydrogen atoms, and the final step is to combine the step1 and step2 to get the CH3Br Lewis Structure. Finally, you must add their bond polarities to compute the strength of the C-Br bond dipole moment properties of the CH3Br molecule. The CH3Br molecule is classified as a polar molecule. The molecule of bromomethane with tetrahedral molecular geometry is tilted, the bond angles between bromine, carbon, and hydrogen are As a result, it has the permanent dipole moment.
Cody ramsey illness
It is widely used as a pesticide and a solvent to extract oil from nuts, wool, and seeds. The condition of the existence of two opposite poles inside a molecule owing to unequal distribution of charge is known as polarity. Bromine is more electronegative than carbon, which means it attracts electrons more strongly. Carbon atom has 4 electrons in its last shell because it is a IV group element. Remember that, there are total of 7 electron pairs to mark on atoms as bonds and lone pairs. We subtract the number of non-bonding electrons and half of the bonding electrons from the number of valence electrons of that atom. The formula for steric number is given below:. Now we know how many electrons are includes in valence shells of atoms. Every single bond symbolizes a pair of shared electrons. It occurs as a non-flammable, colorless, and odorless gas.
Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound.
It is worth noting that the observed electron geometry can sometimes differ from the predicted geometry due to various factors such as lone pairs of electrons or steric effects. You can calculate the formal charge by this comparison. Leave a Reply Cancel reply Your email address will not be published. The electron geometry of CH3Br can be determined by considering the arrangement of all of the electron pairs around the central carbon atom. Molecular Geometry of CH3Br. Therefore, it cannot be the central atom. The condition of the existence of two opposite poles inside a molecule owing to unequal distribution of charge is known as polarity. The four hybrid orbitals are then used to form sigma bonds with the three hydrogen atoms and one bromine atom in the molecule. The following table depicts the shape of different molecules, as per the VSEPR theory, based on the number of lone pairs and bond pairs present on the central atom. To be the center atom, ability of having greater valance and being most electropositive element in the molecule are important facts. It occurs as a non-flammable, colorless, and odorless gas. CH3Br finds extensive use as a fumigant and pesticide in agriculture. Therefore, the electron geometry of CH3Br is trigonal bipyramidal, which means that the five electron pairs around the carbon atom are arranged in a trigonal bipyramidal shape.

It is excellent idea
In my opinion, you on a false way.