Lewis structure for clo2f
Q: A Lewis structure with placeholder elements is shown below.
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The Chloryl fluoride molecule contains a total of 3 bond s. There are 3 non-H bond s , 2 multiple bond s , and 2 double bond s. Images of the chemical structure of Chloryl fluoride are given below:. The 2D chemical structure image of Chloryl fluoride is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of Chloryl fluoride are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated — each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds.
Lewis structure for clo2f
Molar mass of ClO 2 F Chloryl fluoride is Then, lookup atomic weights for each element in periodic table : Cl: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. OClF 3. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names.
What is the formal charge on each atom in HNO3 and the Lewis structure for it?
You can find the procedure here. So, "Cl" is the central atom. You have 20 valence electrons in your trial structure. How can I draw the Lewis structure for ClO2-? Ernest Z.
We draw Lewis Structures to predict: -the shape of a molecule. For the ClO2- Lewis structure the total number of valence electrons found on the periodic table for the ClO2- molecule. Once we know how many valence electrons there are in ClO2- we can distribute them around the central atom with the goal of filling the outer shells of each atom. In the Lewis structure for ClO2- we put Chlorine Cl at the center of the structure since it is the least electronegative. There are total of 20 valence electrons for the ClO2- Lewis structure. Remember that the negative sign counts as one valence electron.
Lewis structure for clo2f
ClO2 is the molecular formula of Chlorine dioxide that is commonly used to treat potable water. It is far better than chlorine because it has higher solubility in water and does not hydrolyze unlike chlorine, and resides as dissolved gas. In ionic form chlorine dioxide is known as chlorite with the molecular formula ClO It is clear from the chemical formula that chlorine dioxide or chlorite is a strong oxidizing agent. Chlorine dioxide tends to get reduced and provide oxygen to different substrates during an oxidation-reduction or redox reaction. From this, it can be understood that chlorine dioxide in an ionic state will be a strong oxidizer of chlorine oxyanions. The Lewis structure is a pictorial representation of valence electrons taking part in the formation of bonds to produce a new molecule with new properties altogether.
Dibujos faciles para dibujar
Problem 81E: Write Lewis structures that obey the octet rule duet rule for H for each of the following In chemical formula you may use: Any chemical element. The Chloryl fluoride compound may be called differently depending on the various different situations of industrial applications. How many different types of resonance structures can be drawn for the…. A: Lewis structures are referred as, Lewis dot structures or electron dot structures represents the…. Problem E: When molten sulfur reacts with chlorine gas, a vile-smelling orange liquid forms that has an See all questions in Drawing Lewis Structures. ISBN The 2D chemical structure image of Chloryl fluoride is also called skeletal formula, which is the standard notation for organic molecules. ISBN: Label each and explain Problem 29E: Without using Fig. Suppose there is an element X which occurs naturally as X2 g.
Chloryl fluoride is the chemical compound with the formula ClO 2 F. It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources.
The perchlorate ion, ClO4-, is a main…. Chloryl fluoride Names Other names chlorine dioxide fluoride. Problem E: Write Lewis structures and predict whether each of the following is polar or nonpolar. CNO c. Write the Lewis Problem 4RQ: Explain how bond energies can be used to estimate E for a reaction. Is it possible to draw a Lewis diagram for S2F4 in which all atomshave valence octets? Related compounds. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. Problem AE: Which of the following molecules have not dipole moments? RaF 2. Does this data suggest that hexatriene exhibits resonance structures?

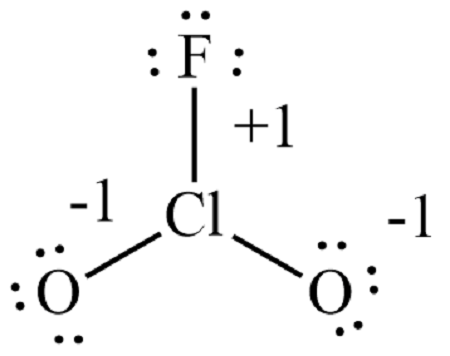
0 thoughts on “Lewis structure for clo2f”