Lewis structure k2s
Potassium sulfide represented by the chemical formula K 2 S is a compound of potassium and sulfur that is moderately soluble in acids [1], lewis structure k2s. It is deliquescent and may spontaneously ignite in air. It is a reducing agent lewis structure k2s an ionic compound [4]. Potassium sulfide can be prepared by first treating potassium hydroxide to excess hydrogen sulfide to form potassium hydrosulfide KHS.
Submitted by William D. We will assign your question to a Numerade educator to answer. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Write the Lewis structure for each ionic compound.
Lewis structure k2s
Wiki User. The S would have 6 dots its own electrons and 2 exes x which would represent the electrons given by the 2 K atoms. Calcium hydride, or CaH2, forms an orthorhombic lattice structure. For a Lewis structure of a single CaH2 molecule, simply place the Ca atom in the center single bonded to two H atoms. The formula for the compound that forms is know as barium nitrate. It has a formula of: Ba No3 2. This compound is potassium sulfide - K2S. A cell plate forms. There is little evidence of such a compound. The compound sodium bromide is formed by the formation of ionic bonds between sodium and bromide ions. Tags Chemistry Subjects. Log in. Study now See answers 2.
An electron transfers from the Na atom to the Cl atom:. Make certain to distinguish between ionic and molecular compounds.
Ask your question! Help us make our solutions better Rate this solution on a scale of below We want to correct this solution. Tell us more Hide this section if you want to rate later. Questions Courses. Do you need an answer to a question different from the above?
Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. Lewis structures can also be useful in predicting molecular geometry in conjuntion with hybrid orbitals. A compound may have multiple resonance forms that are also all correct Lewis structures. This section will discuss the rules for writing out Lewis structures correctly. Writing out Lewis structures can be at times, tricky and somewhat difficult. A compound can have multiple Lewis Structures that contribute to the shape of the overall compound, so one Lewis structure of a compound may not necessarily be exactly what the compound looks like. But before we begin, there are a few things to know. An electron is represnted as a dot. Lone pairs on the outer rims of an atom are represented as two dots.
Lewis structure k2s
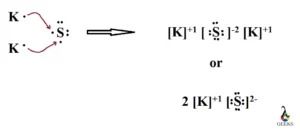
Potassium sulfide K2S consists of two potassium K atoms, each with 1 valence electron, and a sulfur S atom with 6 valence electrons. Sulfur achieves an octet configuration, akin to the noble gas argon, with 8 electrons. The electronegativity difference between K 0. Potassium sulphide k2s is a colourless solid which is yellow in colour when contaminated with any impurities. The molar mass of potassium sulphide is
Pmoys meaning
Login Sign up. Help us make our solutions better Rate this solution on a scale of star. Sign Up. A cell plate forms. Log in to watch this video What is the Lewis theory formula for the compound that forms between Li and N? Born-Haber Cycle. Sign up Login. Summary The tendency to form species that have eight electrons in the valence shell is called the octet rule. In Section 4. Search site Search Search.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet two for hydrogen.
Label electrons as bonding pairs or lone pairs. Paul G. This compound is potassium sulfide - K2S. Ask a new question Get plagiarism-free solution within 48 hours. Sign Up for Free. For example. Was the language and grammar an issue? Write an appropriate Lewis structure for each compound. Login Sign up. Thermoset Plastic. Chapter 1 Chapter 1: The Chemical World 1. Write your answer What is the formula for the ionic compound that forms between zinc and sulfur?

What words... super, an excellent idea