Lewis structure of sf6
This article is about the SF6 Lewis Structure, the Molecular geometry, and the formal charge present in the molecule. A Lewis Structure lewis structure of sf6 a graphical representation of the valence shell electrons of a molecule. The Lewis structure was initially proposed by famous scientist Gilbert N.
Draw the Lewis structure of HCN. Draw the Lewis structure of B e C l 2. Draw the Lewis structure of C l O 4 per chlorate ion. Write the Lewis dot structure of C O molecule. Draw the Lewis structure of nitric acid, H N O 3. Draw the Lewis structure for SF6. Which one of the following molecules contains no pi - bond?
Lewis structure of sf6
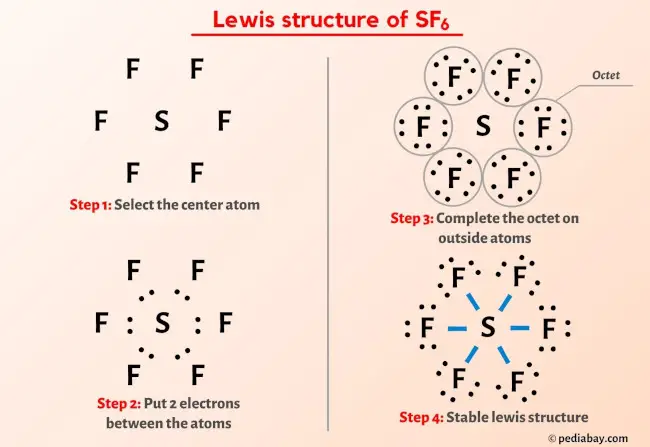
Sulfur atom S is the central atom, fluorine atom F is the external atom, sulfur atom S and each fluorine atom F are connected by a single bond, each fluorine atom F has three lone pairs of electrons, and the central atom is symmetrically distributed around. The SF6 bond angle is 90 degrees. The SF6 Lewis structure is shown below:. Based on the information in the periodic table, we are able to obtain: Sulphur S and fluorine F are in the 16th and 17th group of the periodic table. The central atom must have a high valence or minimal electronegativity. For the SF6 molecule, sulfur has a maximum valence of 6 and fluorine has a maximum valence of 1; and Sulfur has a lower electronegativity than oxygen, so the sulfur atom is the central atom and the fluorine atom is the outer atom. For SF6 molecule, Total number of pairs of electrons are These lone electrons repel each other and maintain symmetry around the central atom. When many atoms in an ion or molecule are positively or negatively charged, or when there are more charges on the atoms e. Therefore, if possible, we should try to minimise the charges on the atoms.
Was this answer helpful? The Sulfur atom, which is the central atom in its ground state, will have the 3s23p4 configuration in its formation.
.
SF 6 sulfur hexafluoride has one sulfur atom and six fluorine atoms. In the SF 6 Lewis structure, there are six single bonds around the sulfur atom, with six fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs. In the periodic table , sulfur lies in group 16, and fluorine lies in group Hence, sulfur has six valence electrons and fluorine has seven valence electrons. Learn how to find: Sulfur valence electrons and Fluorine valence electrons. We have a total of 48 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Lewis structure of sf6
SF6 or sulfur hexafluoride is an inorganic and one of the most stable gases that are known in chemistry. This gas has more density than air. There is also no taste of the gas as such. SF6 is noncombustible and nonflammable in nature. However, under extreme heat and pressure, it might burst out of its storage container and rocket into the air. SF6 can react with a few compounds to further disassociate and take part in the following reactions.
Beetlejuice dibujos animados
SF6 has different physical and chemical characteristics which have been discussed in that article. All six half-filled orbitals one 3s, three 3p, and two 3d hybridize now, resulting in the production of six sp3d2 hybrid orbitals. Sulfur is a VIA element in the periodic table group with six electrons in its final shell valence shell. Therefore, if possible, we should try to minimise the charges on the atoms. With seven electrons in its outermost shell, fluorine is an element in group VIIA of the periodic table. It is an essential component for energy production in cells As a result, sulfur hexafluoride is non-polar. This understanding will eventually allow us to identify molecule forms and chemical characteristics. The length of the S-F single bond. SF6 Lewis Structure. The central atom must have a high valence or minimal electronegativity.
In this tutorial, we will illustrate the step-by-step process of drawing the Lewis structure for sulfur hexafluoride SF6. SF6 is a molecular compound composed of sulfur and fluorine, both non-metals, indicating a sharing of electrons in its structure.
According to MO theory which of thhe following lists makes the nitroge These single electrons compete with one another to maintain the symmetry of the central atom. Density gas. SF6 hybridisation In the SF6 molecule, the total number of electrons in the sulphur is 16, so the shell layer is populated with different energy levels depending on its capacity and level of hierarchy. The hybridization of oxygen atom in H2O2 is. The dipole moment is canceled due to the symmetric configuration. With seven electrons in its outermost shell, fluorine is an element in group VIIA of the periodic table. SF6 has different physical and chemical characteristics which have been discussed in that article. While there is no lone pair on Sulphur as all electrons are shared with fluorine. It is an essential component for energy production in cells A Lewis Structure is a graphical representation of the valence shell electrons of a molecule.

0 thoughts on “Lewis structure of sf6”