Lewis structure seo2
There lewis structure seo2 2 double bonds between the Selenium atom Se and each Oxygen atom O. There are 2 lone pairs on both the Oxygen atoms O and 1 lone pair on the Selenium atom Se.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Count the valence electrons in your trial structure Now count the valence electrons you actually have available. Draw a new trial structure, this time inserting one double bond for each extra pair of electrons:. Calculate the formal charge on each atom.
Lewis structure seo2
The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements. SeO 2 exists as a one-dimensional polymer chain. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid. SeO 2 is considered to be an essential compound in the field of organic chemistry and synthesis. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal. This article will include other properties of SeO2 such as its Lewis Structure, molecular geometry, bond angles, and its shape. SeO 2 comprises of one selenium atom and two atoms of Oxygen. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule. Selenium belongs to group 16 in the periodic table and has an electronic configuration of [Ar]4s 2 3d 10 4p 4. Being in group 6 of the periodic table, Oxygen has six valence electrons and has a valency of Thus, the total number of valence electrons in Selenium Dioxide [SeO 2 ] is given by:.
Now in the SeO2 molecule, lewis structure seo2, you have to put the electron pairs between the selenium atom Se and oxygen atoms O. The chemical formula PCl5 represents the chemical compound Phosphorus Pentachloride. The electron geometry around "Se" is trigonal planar.
.
The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements. SeO 2 exists as a one-dimensional polymer chain. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid. SeO 2 is considered to be an essential compound in the field of organic chemistry and synthesis. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal. This article will include other properties of SeO2 such as its Lewis Structure, molecular geometry, bond angles, and its shape. SeO 2 comprises of one selenium atom and two atoms of Oxygen.
Lewis structure seo2
SeO 2 selenium dioxide has one selenium atom and two oxygen atoms. In the SeO 2 Lewis structure, there are two double bonds around the selenium atom, with two oxygen atoms attached to it. Each oxygen atom has two lone pairs, and the selenium atom has one lone pair. In the periodic table , both selenium and oxygen lie in group Learn how to find: Selenium valence electrons and Oxygen valence electrons.
Grinch christmas decorations
Count the valence electrons in your trial structure Leave a Comment Cancel Reply Your email address will not be published. We have three different structures, differing ONLY in the locations of the electrons. So you have seen the above image by now, right? Selenium dioxide exists as a one-dimensional polymer and the central atom, Selenium, bears the connecting Oxygen atom. For academic purposes, we can determine the molecular geometry and shape for a single component of the polymer chain. The presence of positive and negative formal charges tells us that this may not be the most stable structure for SeO 2. Therefore, the Lewis structure of SeO 2 is given below as:. Unfortunately, the selenium atom is not forming an octet here. Now here the given molecule is SeO2 selenium dioxide and it contains selenium atom Se and oxygen atoms O. It occurs as a polymer chain with pyramidal geometry with Se-O bond lengths of pm and terminal Oxygen bond lengths of pm. In order to check the stability of the central selenium Se atom, we have to check whether it is forming an octet or not. Oxygen is group 16 element on the periodic table. What is the electron dot diagram for carbon? If we compare the electronegativity values of selenium Se and oxygen O then the selenium atom is less electronegative.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. Draw a trial structure by putting electron pairs around every atom until each gets an octet.
SeO 2 comprises of one selenium atom and two atoms of Oxygen. This indicates that the selenium Se and oxygen O are chemically bonded with each other in a SeO2 molecule. As shown above, the central Se does not have an octet as it has only six valence electrons. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal. The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. So here the selenium atom Se is the center atom and the oxygen atoms O are the outside atoms. Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. It is a colorless solid and one of the most available forms Selenium. That will normally be the least electronegative atom "Se". Therefore, the Lewis structure of SeO 2 is given below as:. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand.

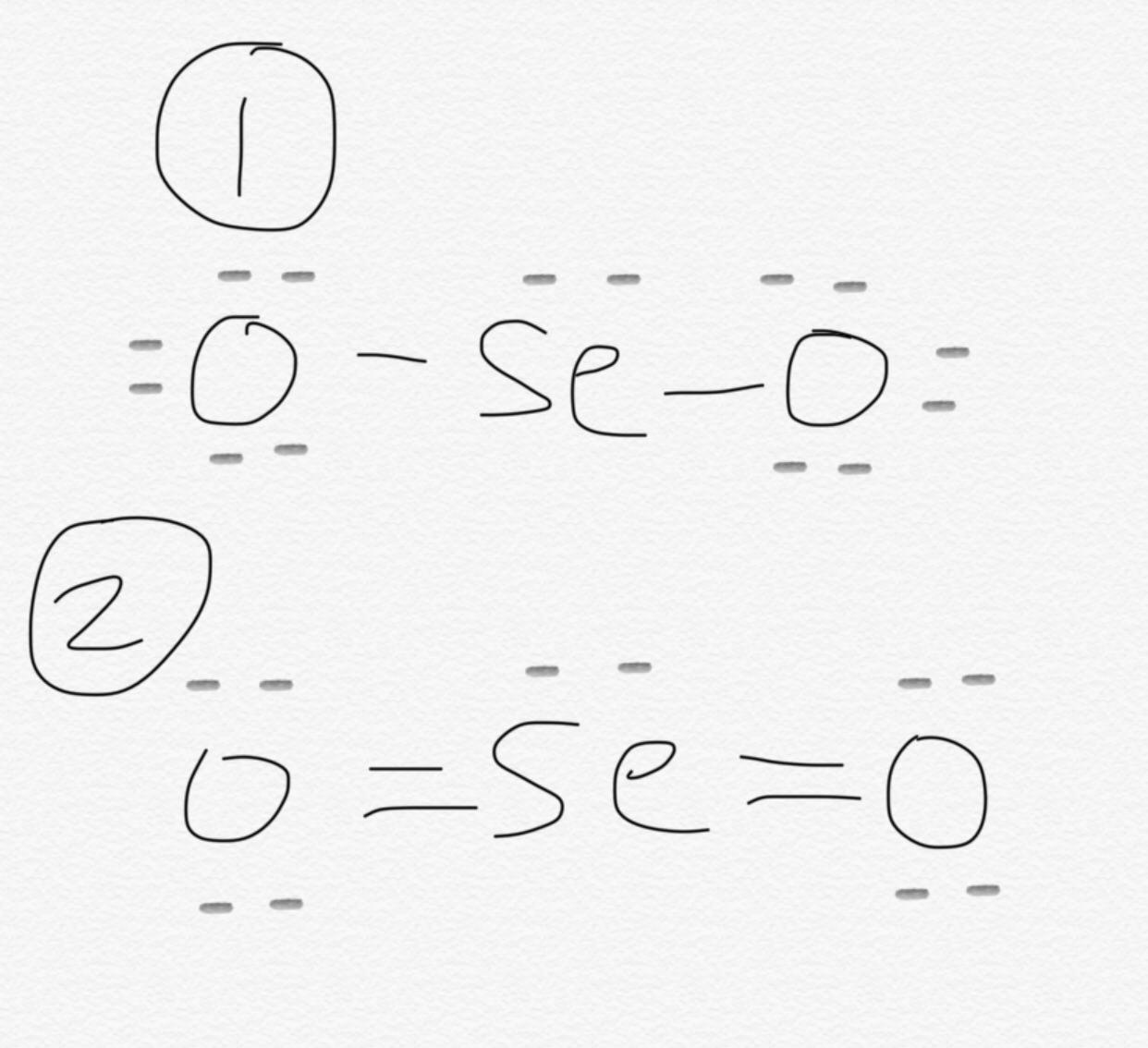
What amusing topic