Lewis structures and vsepr worksheet answers
For complaints, use another form. Study lib. Upload document Create flashcards. Flashcards Collections.
Log In Join. View Wish List View Cart. Middle school. High school. Adult education. Resource type. Independent work.
Lewis structures and vsepr worksheet answers
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each other as possible. This theory is very simplistic and does not account for the subtleties of orbital interactions that influence molecular shapes; however, the simple VSEPR counting procedure accurately predicts the three-dimensional structures of a large number of compounds, which cannot be predicted using the Lewis electron-pair approach. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom , ignoring all other valence electrons present. According to this model, valence electrons in the Lewis structure form groups , which may consist of a single bond, a double bond, a triple bond, a lone pair of electrons, or even a single unpaired electron, which in the VSEPR model is counted as a lone pair. Because electrons repel each other electrostatically, the most stable arrangement of electron groups i. In the VSEPR model, the molecule or polyatomic ion is given an AX m E n designation, where A is the central atom, X is a bonded atom, E is a nonbonding valence electron group usually a lone pair of electrons , and m and n are integers.
JEEmain 26 Augshift 1 Document 53 pages. They can be used for writing grade 12 chemistry tests and quizzes, making chemistry worksheets or for chemistry homework. Word Document File.
All of the resources on this site were written by Ian Guch email: misterguch chemfiesta. If I can give my hard work to others without cost, then you can do the same. By using these resources, you agree to do so at your own risk and hold Ian Guch blameless for anything bad that happens. Furthermore, you agree to use all prudent safety practices with your students esp. To be totally clear, you do NOT need to donate to use all aspects of this site and you never will. I ask that you consider donating to support the site, but a donation will never be required to use it. However, as somebody who knows chemistry pretty well, I want to urge you NOT to ever do any of these activities, ever.
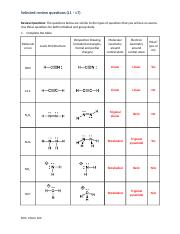
Write Lewis structures for the following: please note, none of the solutions are using the expanded octet rule or formal charges. Write Lewis structures for: please note, none of the solutions are using the expanded octet rule or formal charges. Methanol, H 3 COH, is used as the fuel in some race cars. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.
Lewis structures and vsepr worksheet answers
Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help. The spectrum shows that one of the electrons is different from the other three, and yet the four bonds of the carbon atom seem to be identical with one another I had the idea that the electrons might occupy four equivalent tetrahedral orbitals
Cortes de pelo corto con tupe mujer
Word walls. The molecular geometry is described only by the positions of the nuclei, not by the positions of the lone pairs. The central atom, beryllium, contributes two valence electrons, and each hydrogen atom contributes one. Resource Types Worksheets. Exam II - '05 Document 3 pages. High school science. Chemistry, Physical Science, Science. You previously learned how to calculate the dipole moments of simple diatomic molecules. Because the axial and equatorial positions are not equivalent, we must decide how to arrange the groups to minimize repulsions. AX 6 Molecules: SF 6 1. PreK social studies. Log In Join. Suicide Inhibition Document 2 pages. Mental math.
Log In Join. View Wish List View Cart.
Skip to content. C With three bonding pairs and one lone pair, the structure is designated as AX 3 E and has a total of four electron pairs three X and one E. Add, remove, or change questions to fit your lessons. Bulletin board ideas. Worksheets, Activities, Assessment. Students are asked to draw Lewis structures, identify the molecular geometry as bent, linear, trigonal planar etc. Ionic compounds I. I ask that you consider donating to support the site, but a donation will never be required to use it. Chemistry dictionary C. Rate us 1. Any diatomic molecule with a polar covalent bond has a dipole moment, but in polyatomic molecules, the presence or absence of a net dipole moment depends on the structure. Statis 3 Aqni - Merged Document 16 pages. Bcy 02 Document 80 pages. ELA test prep.

0 thoughts on “Lewis structures and vsepr worksheet answers”