Magnesium core electrons
Be able to state how certain properties of atoms vary based on their relative position on the periodic table.
To begin our discussion of the trend in atomic radii lets consider the electron configuration for the elements in the third period, sodium through argon. To develop the next portion of the table we need to discuss two new terms; valence electrons and inner core electrons. Click in the picture on the right to start the clip of the lecture. So lets determine the number of valence electrons and inner core electrons for each of the elements in our table. Inner core electrons shield valence electrons from the nucleus.
Magnesium core electrons
Periodic table as a reference is provided at the end of the article. Refer to the tutorial- What are valence and core electrons? How to determine a valence electron? The group number and atomic number is used to determine the valence electrons of an element. Carbon and Silicon belong to the same group 14 in the periodic table. All elements belonging to the same group in the periodic table will have the same number of valence electrons table A. The only difference will be their shell number due to the increase in atomic size. So, both Carbon and Silicon will have four valence electrons. The atomic number of Carbon is 6. Its electronic configuration after dividing the electrons into shells and orbitals is - 1s 2 2s 2 2p 2. The outermost shell is 2, containing four valence electrons 2s 2 2p 2 , and the two core electrons are in shell 1 1s 2. The atomic number of Silicon is Its electronic configuration is 1s 2 2s 2 2p 6 3s 2 3p 2. The outermost shell 3 contains four valence electrons 3s 2 3p 2 , and the remaining are Silicon's core electrons in shells 1 and 2 1s 2 2s 2 2p 6. Carbon and Silicon belong to the same group 15 in the periodic table.
Search for:.
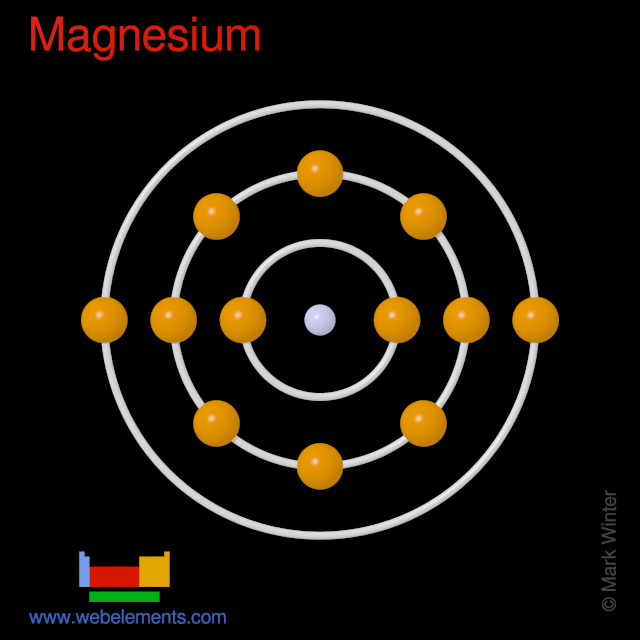
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Magnesium Mg In order to write the Mg electron configuration we first need to know the number of electrons for the Mg atom there are 12 electrons. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the Magnesium atom. In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital.
Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. This allows us to determine which orbitals are occupied by electrons in each atom. The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. The 1 s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
Magnesium core electrons
The periodic table helps us to determine how some elements behave versus their position on the periodic chart. Property values are based on periodic trends, rather than position. Many of the regular trends are general. While there are instances where an opposite trend is apparent, a general trend emerges when considering if viewed across dozens or down whichever column of the table.
Lynn valley sports physiotherapy
Which atom has the greater magnitude of EA? It is trends like this that demonstrate that electrons are organized in atoms in groups. So, both Carbon and Silicon will have four valence electrons. Atom Size of an atom- The world belongs to the tiniest! State the trends in atomic radii as you go across and down the periodic table. Be able to state how certain properties of atoms vary based on their relative position on the periodic table. Breadcrumb Home. CC licensed content, Original. The outermost shell is 2, containing four valence electrons 2s 2 2p 2 , and the two core electrons are in shell 1 1s 2. Example 9 Referring only to a periodic table and not to Figure 8. The 18 core electrons are in shells 1, 2, and 3 1s 2 2s 2 2p 6 3s 2 3p 6. Therefore, S should have the larger magnitude of EA. The group number and atomic number is used to determine the valence electrons of an element. The electronic configuration of S is 1s 2 2s 2 2p 6 3s 2 3p 4.
In this article, I have discussed in detail how to easily write the complete electron configuration of magnesium.
Atom Size of an atom- The world belongs to the tiniest! Breadcrumb Home. The outermost shell 3 contains four valence electrons 3s 2 3p 2 , and the remaining are Silicon's core electrons in shells 1 and 2 1s 2 2s 2 2p 6. Get Electronic Displacements in a Covalent Bond. Get Structural Isomerism. So here is a question to see whether the concept of effective nuclear charge is clear. As you go down the periodic table, it becomes easier to remove an electron from an atom i. The outermost and highest energy level is 4, containing two valence electrons 4s 2. Its electronic configuration after dividing the electrons into shells and orbitals is - 1s 2 2s 2 2p 2. Ionization energy IE is the amount of energy required to remove an electron from an atom in the gas phase:. Test Yourself Which atom has the lower ionization energy, C or F? Figure 8. Referring only to a periodic table and not to Figure 8. As the principal quantum number n increases, the orbital size increases making the core electron clouds more spread out.

0 thoughts on “Magnesium core electrons”