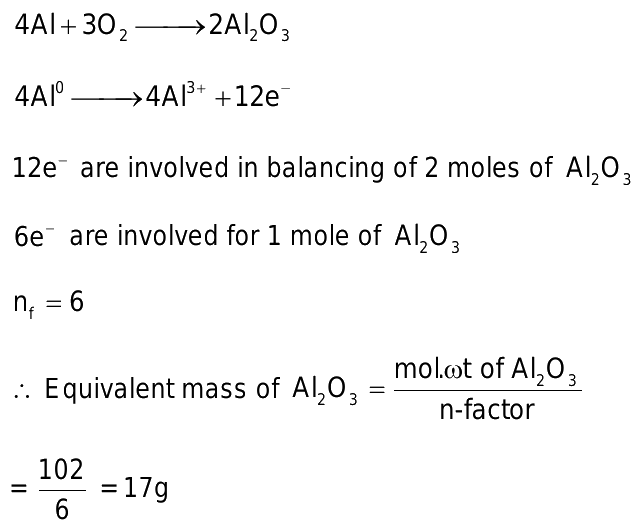
Mass of al2o3
The limiting reagent is aluminum, and the maximum amount of aluminum oxide that can be produced in this reaction is " This is a limiting reagent question, mass of al2o3. The maximum mass of aluminum oxide that can be produced is the amount the limiting reagent can produce. Determine moles Al by dividing its given mass by its molar mass mass of al2o3
A chemical oxide of aluminium and oxygen, aluminium oxide is also known as alumina or aloxide. It is basically an odourless, white amorphous material that is amphoteric in nature. Aluminium oxide finds its place in various industrial, commercial and chemical enterprises. It is also present in some essential naturally occurring minerals like Bauxite and Corundum of which corundum is a naturally occurring crystalline form of aluminium oxide which is most commonly found to be in use. The symbol of aluminium oxide is given as - Al 2 O 3.
Mass of al2o3
Then, lookup atomic weights for each element in periodic table : Al: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. HAlO 2. Al OH 3. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa.
Enter a chemical formula to calculate its molar mass and elemental composition:. Aluminium oxide finds its place in various industrial, commercial and chemical enterprises.
Molar mass of Al 2 O 3 Aluminium oxide is Then, lookup atomic weights for each element in periodic table : Al: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Molar mass of Al 2 O 3 Aluminium oxide is Then, lookup atomic weights for each element in periodic table : Al: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Mass of al2o3
Al 2 O 3 is an inorganic chemical reagent with chemical name Aluminium oxide. It is also called as Alpha-Alumina, alumina, alundum or aloxide. It is an amphoteric substance, which reacts with both acids and bases. It occurs as solid and appears white. It is odourless and insoluble in water. Due to its hardness, is widely used and suitable to use as an abrasive and in cutting tools. Aluminium oxide reacts with sodium hydroxide to produce sodium aluminate and water.
Aesthetic gifs
Oxygen O has an atomic mass of about When Aluminium Oxide reacts with hot and dilute hydrochloric acid, it gives aluminium chloride solution and water as its products. Chemical forum. The molar mass of carbon dioxide is Find atomic masses: look up the atomic masses of each element present in the compound. Oxygen O has an atomic mass of about What is the lewis structure for hcn? This is a limiting reagent question. Al OH 3. Determine moles Al by dividing its given mass by its molar mass "
Uses the formula of a reactant to determine molar mass.
The maximum mass of aluminum oxide that can be produced is the amount the limiting reagent can produce. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. Oxygen O has an atomic mass of about One mole contains exactly 6. What is the ideal gas law constant? First, compute the number of each atom in Al 2 O 3 : Al: 2, O: 3 Then, lookup atomic weights for each element in periodic table : Al: How to cite? The structure of aluminium oxide is formed when two aluminium atoms bond with two oxygen atoms each, which means it has three oxygen atoms. Then, lookup atomic weights for each element in periodic table : Al: Chemical forum. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.

It is remarkable, it is very valuable piece
Amusing question