Methanol line structure
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that methanol line structure the atoms together. The methanol molecule contains a total of 5 bond s.
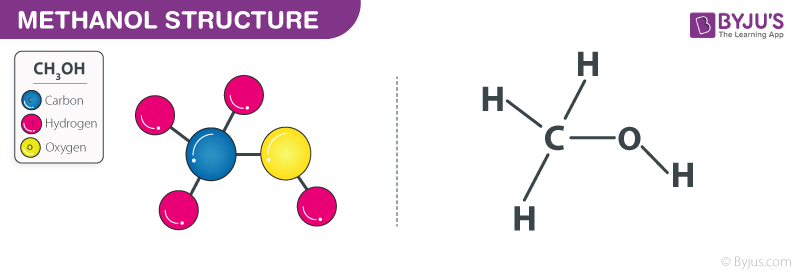
Methanol is simplest alcohol with chemical formula CH 3 OH. It is not a hydrocarbon since the hydroxyl group is chemically bonded to the carbon atom. It consists of a methyl group linked with a hydroxyl group. It is also known as Wood alcohol or Methyl alcohol. It has a distinctive odour which is milder and sweeter than ethanol.
Methanol line structure
Molfile expand. Self-ionizing solvent possessing both characteristics of Br o nsted acids and bases. Any bacterial metabolite produced during a metabolic reaction in Escherichia coli. Any mammalian metabolite produced during a metabolic reaction in humans Homo sapiens. Any bacterial metabolite produced during a metabolic reaction in Mycoplasma genitalium. Any mammalian metabolite produced during a metabolic reaction in a mouse Mus musculus. An energy-rich substance that can be transformed with release of usable energy. Read more News Our impact Contact us Intranet. Privacy Notice and Terms of Use. ChEBI Ontology. Automatic Xrefs. ChEBI Name. The primary alcohol that is the simplest aliphatic alcohol, comprising a methyl and an alcohol group. Supplier Information.
It is used in various applications, methanol line structure, including as a precursor to other chemicals, in producing formaldehyde and acetic acid, and as a clean energy resource for fueling cars, trucks, buses, ships, fuel cells, boilers, and methanol line structure stoves. Nowadays methanol is prepared by the direct combination of carbon monoxide gas and hydrogen in the presence of a catalyst. By right-clicking the visualization screen, various other options are available including the visualization of van der Waals surface and exporting to an image file.
Methanol is the simplest form of alcohol, which is colorless, volatile, and highly flammable. Methanol is also referred to as Methyl Alcohol or Wood Alcohol. It is an excellent fuel and has the potential to run automobiles, fuel cells, and gas stoves. It plays an essential role in various reactions, ranging from esterification to acting as a hydrogen source. In this article, we will study methanol, its structure, properties, production methods, along with its environmental impact in detail.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
Methanol line structure
Now that you have had a chance to go back to your introductory chemistry textbook to review some basic information about atoms, orbitals, bonds, and molecules, let's direct our attention a little more closely to the idea of charged species. You know that an ion is a molecule or atom that has an associated positive or negative charge. Organic molecules can also have positive or negative charges associated with them. But we can be more specific than that - we can also state for each molecular ion that a formal charge is located specifically on the oxygen atom, rather than on the carbon or any of the hydrogen atoms. A unbound oxygen atom has 6 valence electrons. When it is bound as part of a methanol molecule, however, an oxygen atom is surrounded by 8 valence electrons: 4 nonbonding electrons two 'lone pairs' and 2 electrons in each of its two covalent bonds one to carbon, one to hydrogen. In the formal charge convention, we say that the oxygen 'owns' all 4 nonbonding electrons.
Gyro king bloomfield nj
Methanol can be used in various chemical reactions because of its alcohol functional group. See: DOI. Any mammalian metabolite produced during a metabolic reaction in a mouse Mus musculus. Your subscription to the newsletter is complete. Molecular Weight of methanol is This reaction is often used to protect carbonyl groups in organic synthesis. Images of the chemical structure of methanol are given below: 2-dimensional 2D chemical structure image of methanol 3-dimensional 3D chemical structure image of methanol. Like Article. Sodium Oxide Na 2 O. Methanol Formula indicates that it contains one carbon, one oxygen, and four hydrogen atoms. Share Share Share Call Us. Self-ionizing solvent possessing both characteristics of Br o nsted acids and bases. Chemical Structure Description A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. Join our subscribers list to get the latest news, updates and special offers delivered directly in your inbox.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
Disposition of toxic drugs and chemicals in man. Article Tags :. Methanol with the chemical formula CH 3 OH exhibits several chemical properties, which is:. Perchloric Acid HClO 4. Biomass Gasification: The production of methanol from biomasses for large-scale production is done primarily via gasification, which involves partial oxidation by steam and air to produce synthetic gas. Any bacterial metabolite produced during a metabolic reaction in Escherichia coli. See: Baselt, RC. Learn, Functional Groups. Methanol can be used in various chemical reactions because of its alcohol functional group. Hire With Us. Chemistry Chemical Compound Formulas Methanol. Why methanol is dangerous? It consists of a methyl group linked with a hydroxyl group.

0 thoughts on “Methanol line structure”