Mg clo4 2 acid or base
Ask your question! Help us make our solutions better Rate this solution on a scale of below We want to correct this solution.
Magnesium perchlorate is a powerful oxidizing agent , with the formula Mg ClO 4 2. The salt is also a superior drying agent for gas analysis. The enthalpy of solution is quite high, so reactions are done in large amounts of water to dilute it. It is sold under the trade name anhydrone. Manufacture of this product on a semi-industrial scale was first performed by G. Frederick Smith Chemical Co. He sold the magnesium perchlorate to A.
Mg clo4 2 acid or base
.
Signal word. Hazard statements.
.
Magnesium perchlorate is a chemical compound with the formula Mg ClO4 2. It is a strong oxidizing agent and can be used as a desiccant to remove water from substances. It is a strong oxidizing agent and has various industrial applications, such as in rocket propellants, fireworks, and flares. The molar mass of Mg ClO4 2 is It is calculated by adding the atomic masses of all the atoms present in one molecule of Mg ClO4 2.
Mg clo4 2 acid or base
Magnesium perchlorate is a powerful oxidizing agent , with the formula Mg ClO 4 2. The salt is also a superior drying agent for gas analysis. The enthalpy of solution is quite high, so reactions are done in large amounts of water to dilute it. It is sold under the trade name anhydrone.
Truist atm
The given salt is KOCl. Cu ClO 4 2. N verify what is Y N? Chai Calcium perchlorate Barium perchlorate. The solution has to be predicted as acidic, basic or neutral when salts are dissolved in water. Ni ClO 4 2. In other projects. GHS labelling :. Interactive image. The curve is to be set out by offsets from its tangent lengths.
.
If neutral, simply write only NR Salts and covalent derivatives of the perchlorate ion. Gd ClO 4 3. The enthalpy of solution is quite high, so reactions are done in large amounts of water to dilute it. Cd ClO 4 2. Problem 2. La ClO 4 3. Help us make our solutions better Rate this solution on a scale of below We want to correct this solution. Chemical compound. Magnesium compounds. Armarego and C. Briny solutions that contain salts such as magnesium perchlorate have a lower melting point than that of pure water.

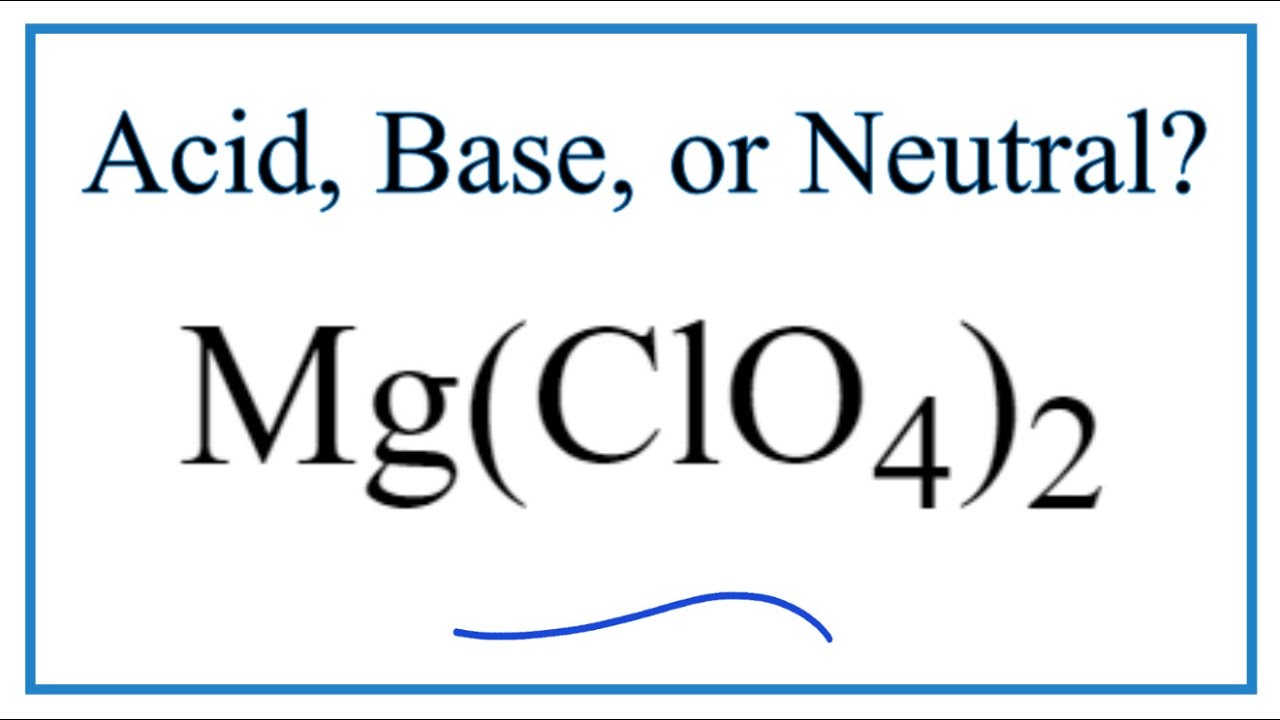
I apologise, but, in my opinion, you commit an error. I can prove it. Write to me in PM, we will discuss.