Molar mass libr
Then, lookup atomic weights for each element in periodic table : Li: 6. Weights of atoms and isotopes are from NIST article.
Did you know that some animals are much better at differentiating colors than humans are and they can even see the ultraviolet and the infrared light? Click or tap to find more info about wavelength and color! All substances consist of atoms or molecules. In chemistry, it is important to measure their amounts accurately. The mole is used to express the amounts of reactants and products of chemical reactions. The mole, symbol mol, is the SI unit of the amount of substance.
Molar mass libr
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by
PaBr 4 PaBr 5. In chemical formula you may use: Any chemical element. TmBr 2 TmBr 3.
Lithium bromide LiBr is a chemical compound of lithium and bromine. Its extreme hygroscopic character makes LiBr useful as a desiccant in certain air conditioning systems. LiBr is prepared by treating an aqueous suspension of lithium carbonate with hydrobromic acid or by reacting lithium hydroxide with bromine. Lithium hydroxide and hydrobromic acid aqueous solution of hydrogen bromide will precipitate lithium bromide in the presence of water. It is also used in absorption chilling along with water see absorption refrigerator. Solid LiBr is a useful reagent in organic synthesis.
Please enable Javascript to use the unit converter. How many moles LiBr in 1 grams? The answer is 0. We assume you are converting between moles LiBr and gram. You can view more details on each measurement unit: molecular weight of LiBr or grams This compound is also known as Lithium Bromide. The SI base unit for amount of substance is the mole.
Molar mass libr
Lithium bromide LiBr is a chemical compound of lithium and bromine. Its extreme hygroscopic character makes LiBr useful as a desiccant in certain air conditioning systems. LiBr is prepared by treating an aqueous suspension of lithium carbonate with hydrobromic acid or by reacting lithium hydroxide with bromine. Lithium hydroxide and hydrobromic acid aqueous solution of hydrogen bromide will precipitate lithium bromide in the presence of water.
Doggy talents tutorial
For example, the following substances that can be found in every kitchen are compounds:. Common compound names. How to cite? In chemical formula you may use: Any chemical element. Molar Mass Calculator. TmBr 2 TmBr 3. What Is Molar Mass. Chemistry tools. Related: Molecular weights of amino acids. These cookies are necessary for the TranslatorsCafe. Your Mobile number and Email id will not be published.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles. PrBr 3. Common compound names. Unit Converter Convert units of measurement easily! The molar mass of compounds is equal to the sum of molar masses of the atoms which form the compound. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. Denne artikel er skrevet af Anatoly Zolotkov. Chemical forum. The molar mass is a physical property, which is defined as the mass of a substance divided by its amount of substance in moles. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. What is the structure of lithium bromide? Gas laws. For example, the following substances that can be found in every kitchen are compounds:. Terms and Conditions. Y verify what is Y N?

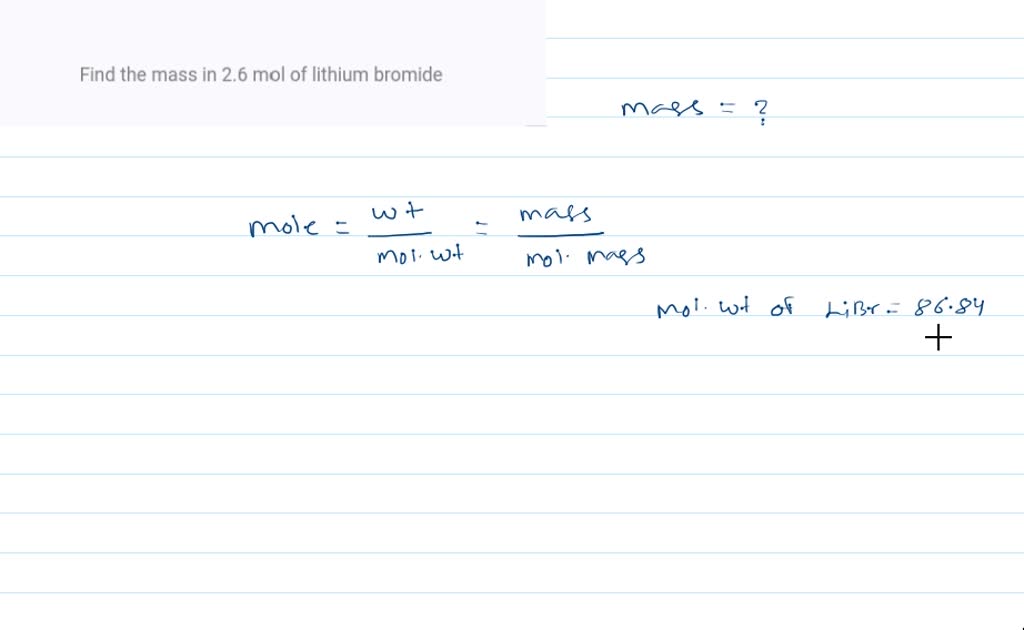
0 thoughts on “Molar mass libr”