Molar mass of k4fe cn 6
Molar mass of K 4 Fe CN 6 is Then, lookup atomic weights for each element in periodic table : K: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
Then, lookup atomic weights for each element in periodic table : K: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Molar mass of k4fe cn 6
This salt forms lemon-yellow monoclinic crystals. In , the French chemist Pierre Joseph Macquer — first reported the preparation of potassium ferrocyanide, which he achieved by reacting Prussian blue iron III ferrocyanide with potassium hydroxide. This solution is then treated with potassium salts to precipitate the mixed calcium-potassium salt CaK 2 [Fe CN 6 ], which in turn is treated with potassium carbonate to give the tetrapotassium salt. Historically, the compound was manufactured from organic compounds containing nitrogen, iron filings, and potassium carbonate. It was also obtained commercially from gasworks spent oxide purification of city gas from hydrogen cyanide. After neutralization of this intermediate with sodium carbonate , red crystals of sodium nitroprusside can be selectively crystallized. Upon treatment with chlorine gas, potassium ferrocyanide converts to potassium ferricyanide :. This reaction can be used to remove potassium ferrocyanide from a solution. A famous reaction involves treatment with ferric salts to give Prussian blue. Potassium ferrocyanide finds many niche applications in industry.
Example: calculating molar mass Let's calculate the molar mass of carbon dioxide CO 2 : Carbon C has an atomic mass of about CO 2 has one carbon atom and two oxygen atoms.
The LabChem potassium ferrocyanide is non-flammable in nature and is mainly used in laboratory and manufacturing factories. Potassium ferrocyanide trihydrate is a salt of the Fe CN ferrocyanide ion. Potassium ferrocyanide is used to determine the concentration of potassium permanganate, a compound often used in…. The Ricca Chemical It is mainly used with road salt and table salt as an anticaking agent.
Molar mass of K 4 Fe CN 6 is Then, lookup atomic weights for each element in periodic table : K: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Molar mass of k4fe cn 6
Then, lookup atomic weights for each element in periodic table : K: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Set up new apple id
In Brauer, G. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. In the EU, ferrocyanides E — were, as of , solely authorised in two food categories as salt additives. Log In or Create a Profile. PMID As soon as these two substances had been mixed together, I saw with astonishment that, without the aid of heat, the blue color had entirely disappeared; the powder [i. Prior to , before the invention of the Castner process , potassium ferrocyanide was the most important source of alkali metal cyanides. Contents move to sidebar hide. From pp. Ullmann's Encyclopedia of Industrial Chemistry.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes.
Refine Results. PubChem CID. Add them together: add the results from step 3 to get the total molar mass of the compound. Upon treatment with chlorine gas, potassium ferrocyanide converts to potassium ferricyanide :. Baker Inc. Other names Yellow Prussiate of Potash, [1] Potassium hexacyanoferrate II trihydrate, Tetrapotassium ferrocyanide trihydrate, Ferrate hexacyano tetrapotassium trihydrate [2]. ISSN In Brauer, G. Gas laws. Potassium ferrocyanide is used in a mixture with potassium ferricyanide and phosphate buffered solution to provide a buffer for beta-galactosidase , which is used to cleave X-Gal , giving a bright blue visualization where an antibody or other molecule , conjugated to Beta-gal, has bonded to its target. Yellow Prussiate of Potash, [1] Potassium hexacyanoferrate II trihydrate, Tetrapotassium ferrocyanide trihydrate, Ferrate hexacyano tetrapotassium trihydrate [2]. This salt forms lemon-yellow monoclinic crystals.

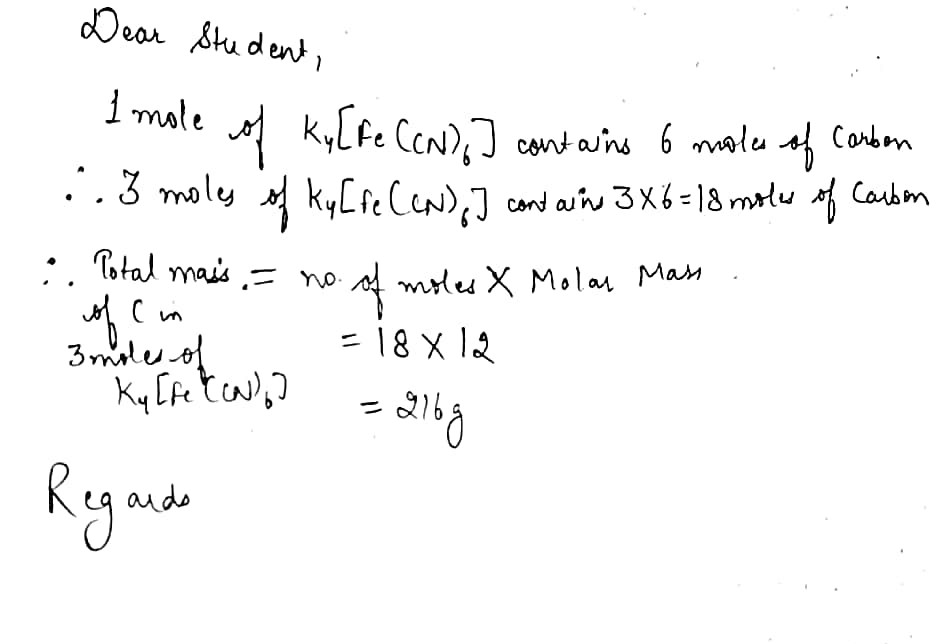
In it something is. Clearly, thanks for an explanation.