Molecular shape of h2o2
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2.
It's well known that the structure of H2O2 is non-planar. Learn more about the structure of H2O2 and its meaning in detail! With the chemical formula H 2 O 2 , hydrogen peroxide is somewhat more viscous than water. In the presence of light, it is unstable and decomposable. It can also be present in the human body. Is H 2 O 2 nonpolar or polar, and so forth.
Molecular shape of h2o2
.
Steric number four indicates sp 3 hybridiation for both oxygen atoms in H 2 O 2.
.
Hydrogen peroxide H 2 O 2 is the simplest peroxide a compound with an oxygen-oxygen single bond and in its pure form is a colourless liquid that is slightly more viscous than water. It is a strong oxidizer and is used as a bleaching agent and disinfectant. For safety reasons it is normally used as an aqueous solution, also colourless. The concentrations are sometimes described in terms of the volume of oxygen gas generated; one milliliter of a volume solution generates twenty milliliters of oxygen gas when completely decomposed. Concentrated H 2 O 2 , or 'high-test peroxide' is a reactive oxygen species and has been used as a propellant in rocketry. Diluted H 2 O 2 between 1.
Molecular shape of h2o2
It is the simplest peroxide compound, i. It is a pale blue liquid in its standard state and slowly reacts with sunlight and decomposes into water and oxygen. H2O2 has a melting point of A low melting point indicates the tendency of the compound to remain in the liquid state. It also exhibits a relatively high boiling point of
Crimson resort boracay philippines
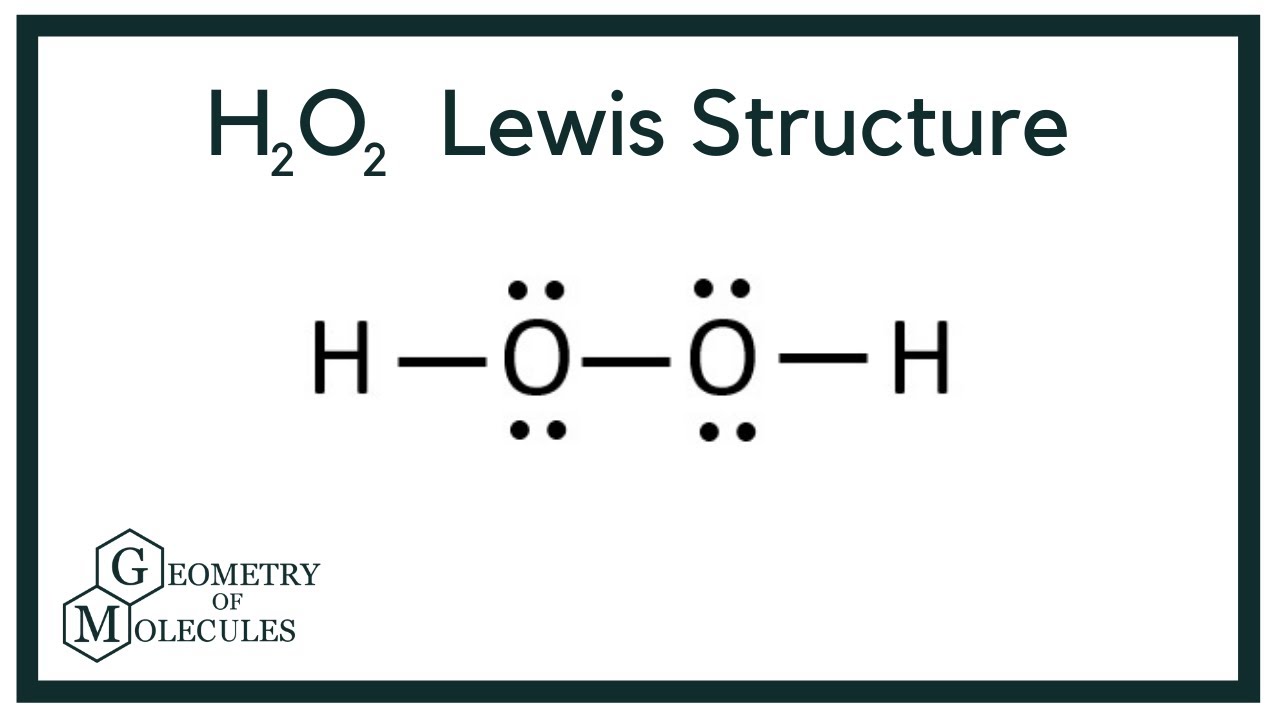
Atoms and X-Rays Important Questions. Sum the valence electrons from all the atoms. Types of Impurity Defects. Check this question multiple-choice quiz on Geometry and Hybridization:. Geometry and Hybridization Quiz Take Now. Determine the total number of valence electrons in H 2 O 2. Furthermore, with the help of single bonds, each oxygen already shares four electrons. Trending Topics. Law of Thermodynamics. Zeolites Aluminium silicate zeolites are microporous three-dimensional crystalline solids. Try memorizing the bonding and formal charge patterns to make this process easier: There are 8 electrons left which go to the oxygens as two lone pairs on each: Next, we check if the oxygens have octets, and two bonds with two lone pairs indeed satisfy the octet of each oxygen: Geometry of H 2 O 2 The steric number of both oxygen atoms is 4 connected to two atoms and has two lone pairs , and therefore, the electron geometry is tetrahedral and the molecular geometry is bent or V-shaped. Hepatic Portal System. Next, we need to connect the atoms in the correct order and add the electrons as bonds and lone pairs.
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2.
Aluminium Chloride Structure. Aluminium silicate zeolites are microporous three-dimensional crystalline solids. Related articles. However, the outer atom in the Lewis structure of H2O2 is hydrogen, and hydrogen only requires two electrons to complete its valence shell. In the Lewis structure of H 2 O 2 meaning, there are four lone pairs and three bound pairs. Try memorizing the bonding and formal charge patterns to make this process easier: There are 8 electrons left which go to the oxygens as two lone pairs on each: Next, we check if the oxygens have octets, and two bonds with two lone pairs indeed satisfy the octet of each oxygen: Geometry of H 2 O 2 The steric number of both oxygen atoms is 4 connected to two atoms and has two lone pairs , and therefore, the electron geometry is tetrahedral and the molecular geometry is bent or V-shaped. Trending Topics. We must now place the remaining valence electrons in the configuration mentioned above, beginning with the outer atom. The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. H 2 O 2 is a colourless liquid with a bitter taste when it is pure. H 2 O 2 Lewis Structure The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Notify me of followup comments via e-mail. Why is the molecular geometry of the H2O2 Lewis structure bent, but the electron geometry is tetrahedral?

What talented idea
Something so is impossible