Moles to moles calculator
Want to know how to calculate moles? Need a grams-to-moles calculator or even a moles-to-grams calculator? Well, then, you've come to the right place. With our moles-to-grams converter, you can seamlessly convert between mass, molecular weight, and moles.
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation. The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients. However, the equation is balanced as long as the coefficients are in a ratio. For example, this equation is also balanced if we write it as.
Moles to moles calculator
Use the mole calculator below to find the quantity of a substance using a chemical formula or known molar mass. Joe is the creator of Inch Calculator and has over 20 years of experience in engineering and construction. He holds several degrees and certifications. Full bio. Teresa is a chemistry expert with a PhD in environmental science, a Master's degree in earth, atmospheric, and planetary sciences, and Bachelor's degree in chemistry. The mole , abbreviated mol , is the SI base unit to measure the amount or quantity of a substance. One mole is equal to 6. Since the mole is a measure of quantity, all substances have the same number of atoms, molecules, and other particles in one mole of the substance. You might be wondering how you can calculate the number of moles given a mass, volume, or amount of substance. This conversion is useful because most chemicals are stored and measured either by weight g or volume L. However, most chemical equations are written in terms of moles so that the number of atoms of each type balances out on both sides of the equation.
List other known quantities.
Our molar ratio calculator will help you determine the molar ratio between the different chemicals reacting and the different chemicals produced during the reaction. It can also help you determine the mass or the number of moles of each chemical required to perform the reaction. Equipped with that knowledge, you can find the limiting agent in the reaction or which chemical is available in excess. Are you wondering what is a molar ratio and how to calculate it? Do you what to learn the importance of a molar ratio?
This Mole Calculator finds the quantity of a substance in moles and molar mass of the substance using its chemical formula and known mass of the substance in grams. Indices should be entered as normal numbers after the appropriate elements or groups, e. H2O for a water molecule. Nested brackets are also allowed, e. The degree of nesting is unlimited but all the brackets should be balanced. You can enter a formula manually or paste the formula copied from a web page or text document including DOC or PDF file. One mole is defined to contain exactly 6. This number known as Avogadro number was chosen so that the mass of one mole of a chemical compound in grams is numerically equal, for most practical purposes, to the average mass of one molecule of the compound in daltons and roughly equivalent to the number of nucleons protons or neutrons in the molecule.
Moles to moles calculator
You can easily add Chemistry Moles Calculator to your own website with the help of our code. Paste the code to your website and the calculator will appear on that spot automatically! Adding Chemistry Moles Calculator to your Wordpres website is fast and easy! Find the page to which you want to add the calculator, go to edit mode, click 'Text', and paste the code to there. The Gibbs energy calculator is the ideal tool for determining whether or not a chemical reaction can happen on its own. This calculator converts the mass concentration of any solution into molar concentration. It also recalculates grams per ml to moles. It is also possible to calculate the mass of any substance required to reach a desired level of molarity.
Download coursehero pdf free
First you must calculate the number of moles in this solution, by rearranging the equation No. For example, we've seen that the molar ratio equation between hydrogen and oxygen in water production is Second reactant. How do you find molar ratio using this online molar ratio calculator? What is the molar ratio between sodium and chlorine in the formation of table salt? Boiling Point Calculator. The lesson? The ionic strength calculator is a convenient tool to help you calculate the ionic strength of a solution based on the ions present in it and their charge. We can also phrase that oxygen is in excess by 4 mol since only 6 mol can react with 12 mol of hydrogen. To produce Books vs e-books Calculator Discover the ultimate paper books vs. Here, the solution being used is typically defined by its molar concentration M.
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation.
With our moles-to-grams converter, you can seamlessly convert between mass, molecular weight, and moles. Use our avogadro's number calculator to understand this further. Repeat step one for the second substance to obtain the number of moles used or produced in the reaction. Atomic mass m a — mass of one atom of that element which typically reflects the mass of the nucleus protons plus neutrons. You can find more information and formulas about grams to moles conversion as well as about moles to grams conversion below the calculator. Well, then, you've come to the right place. The empirical formula indicates the ratio of atoms of each element in a molecule rather than the actual number. This convention is used for most calculations involving gasses and is intended to represent conditions at sea level. For sodium chloride NaCl they are in a ratio of so the molar mass of NaCl is The ionic strength calculator is a convenient tool to help you calculate the ionic strength of a solution based on the ions present in it and their charge. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: standard relative atomic masses are dimensionless quantities i. Make an informed choice with our handy tool. Summary The balanced chemical reaction can be used to determine molar relationships between substances.

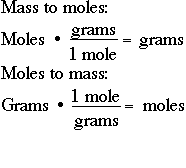
I think, that you are mistaken. I can defend the position. Write to me in PM, we will talk.