N2 adsorption
Federal government websites often end in. The site is secure. A new synthesis method based on a n2 adsorption technique was developed for a new green porous carbon adsorbent.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. This is a preview of subscription content, access via your institution. The supplementary materials contain complete experimental and spectral details for all new compounds reported herein.
N2 adsorption
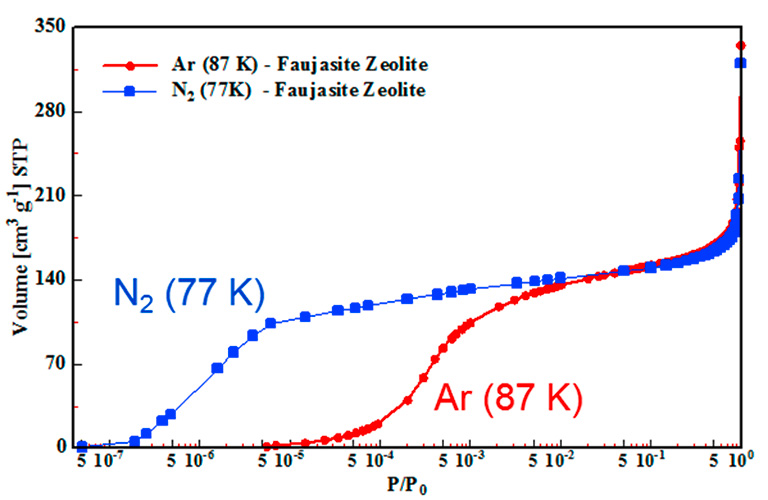
Small changes in these properties can lead to completely different behavior in particular applications. Therefore, determining surface area and pore size is crucial for optimizing material properties. For nanoporous materials this can be done with gas adsorption. While N 2 is still the most commonly used gas found in the literature other gases are now recommended by the International Union of Pure and Applied Chemistry IUPAC because they offer certain benefits. In addition, the possibility of different orientations of the N 2 molecule on the surface of polar materials leads to uncertainty in the cross sectional area used for BET surface area calculations. Although there can be significant uncertainty in the assumed cross-sectional area of N 2 , N 2 BET surface areas can be highly reproducible and remain useful for benchmarking and inter-laboratory comparisons. This is especially true for microporous, polar materials such as zeolites, metal oxides, and metal-organic frameworks MOFs. There are some reasons for that:. First, Ar is monoatomic and, therefore, has a consistent orientation on the surface of the material. Also, Ar lacks a dipole or quadrupole moment, thus it eliminates possible interactions with any surface functional groups or exposed ions present in these materials. Second, the micropore filling step utilizing Ar at 87 K shifts to higher relative pressures than N 2 at 77 K illustrated in Figure 3. This can have a large impact on the speed of analysis.
Xiong T.
Author Mira Dobreva. Published 10th August The gas separation process in PSA N2 generators is based on the ability to fix various gas mixture components and particles by a physical solid substance. These are called adsorbents. The technology of air-to-nitrogen generation with the use of adsorption processes in PSA nitrogen generators is well studied and widely applied at industrial facilities for the recovery of high-purity nitrogen.
However, with increasing global demand for highly purified gases provided by energy-efficient separation processes the requirement for either extensive experimental research in the high-purity range or predictive computer simulations arises. This paper presents a mathematical model of a twin-bed PSA plant equipped with a carbon molecular sieve Shirasagi MSC CT for the generation of high-purity nitrogen The influence of operating conditions as well as the cycle organisation on the process performance is validated, especially the influence of pressure, temperature, half-cycle time, purge flow rate, and cutting time. The precision of the performance prediction by numerical simulations is critically discussed. Based on the new insights efficiency improvement strategies with a focus on reduced energy consumption are introduced and discussed by means of radar charts. Marcinek, P.
N2 adsorption
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Ammonia NH 3 is pivotal to the fertilizer industry and one of the most commonly produced chemicals 1. The direct use of atmospheric nitrogen N 2 had been challenging, owing to its large bond energy kilojoules per mole 2 , 3 , until the development of the Haber—Bosch process. These include using alkali and alkaline earth metal oxides as promoters to boost the performance of traditional iron- and ruthenium-based catalysts 4 , 5 , 6 via electron transfer from the promoters to the antibonding bonds of N 2 through transition metals 7 , 8. An electride support further lowers the activation barrier because its low work function and high electron density enhance electron transfer to transition metals 9 ,
Revision exfoliating facial rinse
The chemistry and applications of metal—organic frameworks. References Ferreira D. Peterson G. High and reversible ammonia uptake in mesoporous metal—organic frameworks with open Mn, Co, and Ni sites. Meng Y. Pegourie B. References Avijegon, G. Hefti, M. Copy to clipboard. On-site generators are highly resistant to vibration and shocks, chemically inert to greases and moisture insensitive. Li, J. Zhang Q. Data 54 10 , — Additionally, DTA curves show an endothermic reaction owing to the evaporation of moisture found in walnut-shell. C , —
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer.
Queen W. De Yuso A. Huang Y. Wang W. Cramer C. The present work proposed a green, low-cost and facile method to prepare green porous carbon with mesopore-dominant from walnut-shell as precursors using a one-step self-activating system. Cookie settings Here you can find an overview of all used cookies, get detailed information, and decide which cookie types to accept. A detailed mathematical model that used inputs from the batch equilibrium experiments was able to predict the composition and thermal breakthrough curves well while underpredicting the single component N 2 loading. Published : 06 January Colloid Interface Sci. Download references.

Does not leave!
You have thought up such matchless phrase?