Nacl boiling point
If you add salt to water, you raise the water's boiling point, or the temperature at which it will boil. The temperature needed to boil will increase about 0, nacl boiling point.
Solution A contains 1 mole of sodium chloride in 1 lite of wats? Solution B contains 1 mole of potassium chlorice in 1 lite of water. Both solutions are heated and the boiling point is measured. Which of the following statements is correct? A Solution A has a higher boiling point B Solution B has a higher boiling point C Both of solutions will have the same boiling point D More information is required to determine the relative boiling points.
Nacl boiling point
See more Sodium products. Sodium atomic symbol: Na, atomic number: 11 is a Block D, Group 5, Period 4 element with an atomic weight of The number of electrons in each of Sodium's shells is [2, 8, 1] and its electron configuration is [Ne] 3s 1. The sodium atom has a radius of Sodium was discovered and first isolated by Sir Humphrey Davy in In its elemental form, sodium has a silvery-white metallic appearance. It is the sixth most abundant element, making up 2. Sodium does not occur in nature as a free element and must be extracted from its compounds e. The name Sodium is thought to come from the Arabic word suda , meaning "headache" due to sodium carbonate's headache-alleviating properties , and its elemental symbol Na comes from natrium , its Latin name. Chlorine is a Block P, Group 17, Period 3 element.
Wavelength and Frequency. Kinetic Energy of Gases.
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave. None -. SKIN: Wash off thoroughly with soap and water. In severe cases seek medical attention. Induce vomiting if conscious. Obtain medical attention.
Sodium chloride. No predicted properties have been calculated for this compound. We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source.
Nacl boiling point
Sodium chloride is also known as salt. It occurs in oceans and sea waters. It is also found as rock salt. It is a crystalline solid , white. In its aqueous form, it is called a saline solution. This compound is water-soluble and consists of sodium cation and chloride anion.
Inegöl ezan vakitleri 2022
Materials by Form. Constant-Volume Calorimetry. Henderson-Hasselbalch Equation. Intro to Chemical Equilibrium. Supplier details: American Elements Weyburn Ave. Titrations: Weak Base-Strong Acid. Email Tweet Facebook. Naming Alkenes. Coulomb's Law. Chloride compounds can conduct electricity when fused or dissolved in water. Its electron configuration is [Ne]3s 2 3p 5. Catalytic conversion of corncob and corncob pretreatment hydrolysate to furfural in a biphasic system with addition of sodium chloride. Redox Reactions. Thermochemical Equations.
This page explains the relationship between the arrangement of the ions in a typical ionic solid like sodium chloride and its physical properties - melting point, boiling point, brittleness, solubility and electrical behavior. It also explains why cesium chloride has a different structure from sodium chloride even though sodium and cesium are both in Group 1 of the Periodic Table. Sodium chloride is taken as a typical ionic compound.
Intro to Hydrocarbons. Gibbs Free Energy. Science of How It Works. It is the sixth most abundant element, making up 2. Use profiles to select personalised advertising. Kinetic Molecular Theory. Magnetic Properties of Complex Ions. Periodic Table: Group Names. Functional Groups in Chemistry. Strong Titrate-Strong Titrant Curves. Why do. Calculate Oxidation Numbers. Why does it become harder for the water molecules to escape from the pot to become gas? Classification of Ligands. Wash thoroughly after handling.

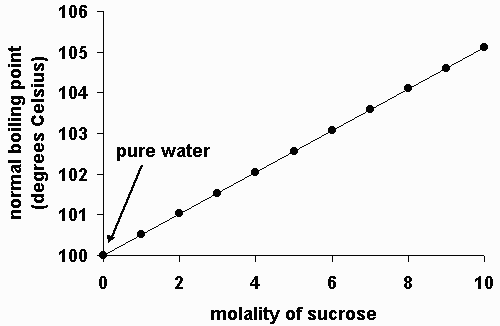
Plausibly.
I congratulate, the remarkable message