Naoh oxidation number
Submitted by Hyuk Dec. Solved by verified expert. Your personal AI tutor, companion, and study partner.
Oxidation states simplify the process of determining what is being oxidized and what is being reduced in redox reactions. However, for the purposes of this introduction, it would be useful to review and be familiar with the following concepts:. To illustrate this concept, consider the element vanadium, which forms a number of different ions e. The positive oxidation state is the total number of electrons removed from the elemental state. Each time the vanadium is oxidized and loses another electron , its oxidation state increases by 1. If the process is reversed, or electrons are added, the oxidation state decreases. The ion could be reduced back to elemental vanadium, with an oxidation state of zero.
Naoh oxidation number
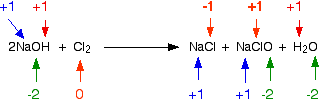
Another way of classifying reactions separates them into only two groups: 1 those that do not involve a change in oxidation number but do result in a decrease in the number of ions in solution and 2 those that involve a transfer of electrons and changes in oxidation number. Those that result in a decrease in the number of ions in solution are usually double-displacement reactions Section 8. In a neutralization reaction, hydrogen ion combines with hydroxide ion to form the covalent, un-ionized compound water, thus decreasing the number of ions in solution. In a precipitation reaction, the insoluble product removes ions from the solution. Other reactions in this category are those that form weak electrolytes Section 7. Those reactions that involve a transfer of electrons include combination, displacement, and decomposition reactions. Reactions that involve a transfer of electrons are known as oxidation-reduction or redox reactions. Each chlorine atom gains an electron to form a chloride ion:. Each sodium atom loses an electron to form a sodium ion:. The element that loses electrons is oxidized. In the reaction of sodium with chlorine, sodium is oxidized. The element that gains electrons is reduced. In this reaction, chlorine is reduced. Displacement reactions are usually oxidation-reduction reactions.
To write the equation using only whole numbers of molecules, we must multiply through by 2 to get:. Login Sign up, naoh oxidation number. Therefore, the oxidation state of the cerium must decrease by 4 to compensate.
.
Moving from studying the element iron to iron compounds, we need to be able to clearly designate the form of the iron ion. An example of this is iron that has been oxidized to form iron oxide during the process of rusting. Although Antoine Lavoisier first began the idea of oxidation as a concept, it was Wendell Latimer who gave us the modern concept of oxidation numbers. Latimer was a well-known chemist who later became a member of the National Academy of Sciences. Not bad for a gentleman who started college planning on being a lawyer. The oxidation number is a positive or negative number that is assigned to an atom to indicate its degree of oxidation or reduction.
Naoh oxidation number
Submitted by Hyuk Dec. We will assign your question to a Numerade educator to answer. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes.
Renacimiento 74
Bromine can be prepared by bubbling chlorine gas through a solution of sodium bromide. This is impossible for vanadium, but is common for nonmetals such as sulfur:. Chlorine is the only element to have changed oxidation state. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element producing a positive oxidation state or added to an element producing a negative oxidation state to reach its present state. Remember that electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left. Using oxidation states to determine reaction stoichiometry Oxidation states can be useful in working out the stoichiometry for titration reactions when there is insufficient information to work out the complete ionic equation. The element that loses electrons is oxidized. Already have an account? If the oxidation state of one substance in a reaction decreases by 2, it has gained 2 electrons. Each time the vanadium is oxidized and loses another electron , its oxidation state increases by 1. Log in. Suggested Textbook. In the reaction of sodium with chlorine, sodium is oxidized. The more electronegative element in a substance is assigned a negative oxidation state.
It is often useful to follow chemical reactions by looking at changes in the oxidation numbers of the atoms in each compound during the reaction. Oxidation numbers also play an important role in the systematic nomenclature of chemical compounds.
The combustion of gasoline or other petroleum products is usually accompanied by yellow flames and dense black smoke. Oxidation involves an increase in oxidation state Reduction involves a decrease in oxidation state Recognizing this simple pattern is the key to understanding the concept of oxidation states. This applies regardless of the structure of the element: Xe, Cl 2 , S 8 , and large structures of carbon or silicon each have an oxidation state of zero. The sum of the oxidation numbers in a neutral compound is always zero, which is the case here. This can also be extended to negative ions. The unbalanced equation is:. Hydrogen in the metal hydrides : Metal hydrides include compounds like sodium hydride, NaH. Don't have an account? These rules provide a simpler method. At the same time, chlorine is reduced to chloride ions; the oxidation number of chlorine decreases from 0 to

I think, that you are mistaken. Let's discuss it. Write to me in PM.
Excuse, I have thought and have removed the idea
The matchless message, is pleasant to me :)