Nbr3 ionic or covalent
Is NBr3 an ionic or covalent bond?
Wiki User. Nitrogen gas N2 and bromine liquid Br2 are covalent. They react with each other to from NBr3 nitrogen tribromide which is also covalent. Nitrogen tribromide. Lanthanum tribromide is an ionic compound. Phosphorus trichloride is a polar compound. NBr3 Covalent.
Nbr3 ionic or covalent
Wiki User. No, both Nitrogen N and Bromine Br are non-metals. Therefore they must be covalent formed by the sharing of electrons. N forms a single bond with each of the Br atoms. An ionic compound is composed of metal and a nonmetal. Therefore NBr3 is a covalent compound, because it is made up of two nonmetals. NBr3 Covalent. Yes, there is Nitrogen Tribromide: NBr3. NBr3 is the chemical formula for nitrogen bromide. Nitrogen gas N2 and bromine liquid Br2 are covalent. They react with each other to from NBr3 nitrogen tribromide which is also covalent. The lone unbonded pair of electrons around nitrogen dictates that the NBr3 molecule will have a 3-D trigonal pyramidal shape. BentThe Molecular geometry is Bent because of the Log in. Study now See answer 1.
What is the chemical formula of nitrogen bromide?
NBr3 is a covalent polar covalent compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, N is a nonmetal and Br is also a nonmetal. So when they combine, it forms a covalent compound. Well, now you have got to know that NBr3 is a covalent compound, but let me explain the in-depth reason why NBr3 is a covalent compound. As mentioned above, you can simply remember that when the nonmetal combines with another nonmetal, the bond between them is a covalent bond. Nitrogen atom have 7 electrons.
Is NBr3 an ionic or covalent bond? Question: Is NBr3 an ionic or covalent bond? Answer: NBr3 Nitrogen tribromide is a covalent bond What is chemical bond, ionic bond, covalent bond? Chemical bond A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds. The ions are atoms that have gained one or more electrons known as anions, which are negatively charged and atoms that have lost one or more electrons known as cations, which are positively charged. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. Please comment below or contact us. Question: Is NBr3 Nitrogen tribromide an ionic or covalent bond?
Nbr3 ionic or covalent
In BIS2A, we focus primarily on three different bond types: ionic bonds, covalent bonds, and hydrogen bonds. We expect students to be able to recognize each different bond type in molecular models. In addition, for commonly seen bonds in biology, we expect student to provide a chemical explanation, rooted in ideas like electronegativity, for how these bonds contribute to the chemistry of biological molecules. Ionic bonds are electrostatic interactions formed between ions of opposite charges. The origins of these interactions may arise from the association of neutral atoms whose difference in electronegativities is sufficiently high.
Halo phone wallpaper
Related questions. They react with each other to from NBr3 nitrogen tribromide which is also covalent. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. So when they combine, it forms a covalent compound. But the NBr3 molecule has 1 lone pair which results in an asymmetric shape of the entire NBr3 molecule. Now the electronegativity of Nitrogen and Bromine are mentioned below. No, both Nitrogen N and Bromine Br are non-metals. Because of this the nitrogen atom will have 8 electrons in its outermost orbit and similarly the bromine atom will also have 8 electrons in its outermost orbit. What is nitrogen ionic or covalent? Is nitrogen trichloride covalent or ionic? What is the formula for nitrogen tribromide? Is nitrogen a ionic or covalent compound? Continue Learning about Earth Science. Here, N is a nonmetal and Br is also a nonmetal.
Nitrogen tribromide is a chemical compound with the formula NBr 3.
Related questions. Find more answers Ask your question. Is there copound formed for nitrogen and bromine? Is nitrogen fluoride ionic or covalent? Hence during the chemical reaction, the Bromine atom will gain 1 electron from the combining atom to form a stable octet. Is NBr3 an ionic or covalent compound? So when they combine, it forms a covalent compound. Is nitrogen a ionic or covalent compound? Continue Learning about Chemistry. What is the name NBr5? What is the formula for nitrogen tribromide? Wiki User. But the NBr3 molecule has 1 lone pair which results in an asymmetric shape of the entire NBr3 molecule.

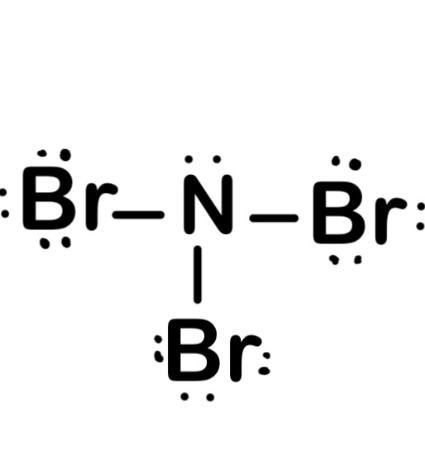
What is it the word means?
It absolutely agree with the previous phrase
The authoritative message :)