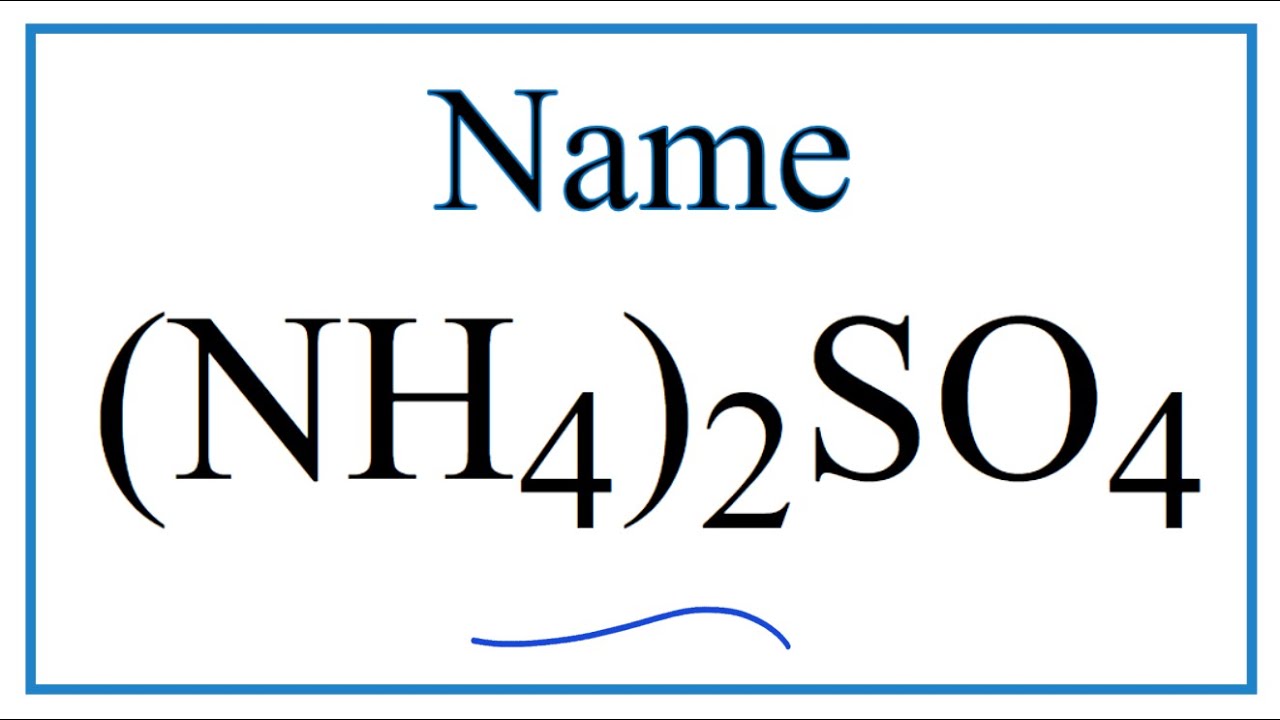
Nh4 2so4
Ammonium Sulfate is non-hazardous to humans. It is also known as Diammonium sulfate or Sulphuric acid diammonium salt. It has no smell and dissolves in nh4 2so4 easily.
Ammonium Sulfate has a wide range of uses providing a good source of both sulfur and nitrogen. To report an issue with this product or seller, click here. Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon. It also analyzed reviews to verify trustworthiness. Customers like the quality, ease of use, growth, and speed of the lab chemical.
Nh4 2so4
Ammonium sulfate American English and international scientific usage; ammonium sulphate in British English ; NH 4 2 SO 4 , is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. The primary use of ammonium sulfate is as a fertilizer for alkaline soils. In the soil, the ammonium ion is released and forms a small amount of acid, lowering the pH balance of the soil , while contributing essential nitrogen for plant growth. The main disadvantage to the use of ammonium sulfate is its low nitrogen content relative to ammonium nitrate , which elevates transportation costs. It is also used as an agricultural spray adjuvant for water-soluble insecticides , herbicides , and fungicides. There, it functions to bind iron and calcium cations that are present in both well water and plant cells. It is particularly effective as an adjuvant for 2,4-D amine , glyphosate , and glufosinate herbicides. Ammonium sulfate precipitation is a common method for protein purification by precipitation. As the ionic strength of a solution increases, the solubility of proteins in that solution decreases. Ammonium sulfate is extremely soluble in water due to its ionic nature, therefore it can "salt out" proteins by precipitation. The significance of this substance in the purification of compounds stems from its ability to become more so hydrated compared to relatively more nonpolar molecules and so the desirable nonpolar molecules coalesce and precipitate out of the solution in a concentrated form. This method is called salting out and necessitates the use of high salt concentrations that can reliably dissolve in the aqueous mixture. The percentage of the salt used is in comparison to the maximal concentration of the salt in the mixture can dissolve. Precipitation by ammonium sulfate is a result of a reduction in solubility rather than protein denaturation , thus the precipitated protein can be solubilized through the use of standard buffers.
Additional Details.
.
Diammonium sulfate. No predicted properties have been calculated for this compound. We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record.
Nh4 2so4
Ammonium Sulfate is non-hazardous to humans. It is also known as Diammonium sulfate or Sulphuric acid diammonium salt. It has no smell and dissolves in water easily. It does not dissolve in acetone. It appears as a crystalline solid white and has a salty taste. Ammonium sulfate is mainly used as a fertilizer for alkaline soils. The reaction between the sulphuric acid and the ammonium hydroxide produces ammonium sulfate.
Stokastic twitter
Came fast. Retrieved 6 June How to return the item? We used this on sweet corn and the corn is growing great so I do recommend this product. Whenever your leaves sag and depressurize and look dried out or deep red and drooping. Sorry we couldn't load the review. Other cations. Ammonium sulfate becomes ferroelectric at temperatures below — How customer reviews and ratings work Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them. Help others learn more about this product by uploading a video! Not dark green and red. Image Unavailable Image not available for Color:. View Result. And right means HIGH.
Ammonium sulfate American English and international scientific usage; ammonium sulphate in British English ; NH 4 2 SO 4 , is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer.
Double metal sulfates include ammonium cobaltous sulfate, ferrous diammonium sulfate , ammonium nickel sulfate which are known as Tutton's salts and ammonium ceric sulfate. Germany Japan. Dissolves instantly. Very Pure Nitrogen Source. It is particularly effective as an adjuvant for 2,4-D amine , glyphosate , and glufosinate herbicides. Gravimetric Analysis. To see product details, add this item to your cart. Nitrogen is the most rapidly depleted. Ammonium sulphate Ammonium sulfate Diammonium sulfate Sulfuric acid diammonium salt Mascagnite Actamaster Dolamin. What type of compound is ammonium oxalate?

What remarkable topic
Really and as I have not guessed earlier