Nh43po4
Then, lookup atomic weights for each nh43po4 in periodic table : P: Weights of atoms and isotopes are from NIST article, nh43po4. WebQC is a web application with nh43po4 mission to provide best-in-class chemistry tools and information to chemists and students.
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH4 3PO4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transform the ammonium phosphate to the molten state. It includes a group of nitrogen phosphorus materials: mono ammonium phosphates and diammonium phosphates, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate. Ammonium phosphate is used as a leavening agent during the process of bakingIts interaction with heat, results in instant evaporation without leaving any residue of ammonia.
Nh43po4
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH 4 3 PO 4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transform the ammonium phosphate to the molten state. It includes a group of nitrogen phosphorus materials: mono ammonium phosphates and diammonium phosphates, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate. Other names — triammonium phosphate, diazanium hydrogen phosphate. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers. This is also used in thermoplastic formulations as a flame retardant. Ammonium phosphate adds nitrogen and phosphates to the lawns which lack the nutrients. Ammonium phosphate is a fast-release fertilizer that can be used for new grass planting, cleaning, monitoring, or lawn renovation. Ammonia contains one nitrogen and three hydrogens opposed to one nitrogen and four hydrogens formed by ammonium. Ammonia is a low, unionized foundation. In the other side, it is ionized to ammonium. Some major distinctions between the two is that Ammonia gives off a heavy odour while Ammonium does not smell at all. Ammonium phosphate is an orthophosphoric acid ammonium salt.
Related: Molecular weights of amino acids. Nh43po4 Edit View history. Contact us.
Ammonium phosphate is the inorganic compound with the formula NH 4 3 PO 4. It is the ammonium salt of orthophosphoric acid. A related "double salt", NH 4 3 PO 4. NH 4 2 HPO 4 is also recognized but is impractical to use. Both triammonium salts evolve ammonia. In contrast to the unstable nature of the triammonium salts, the diammonium phosphate NH 4 2 HPO 4 and monoammonium salt NH 4 H 2 PO 4 are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus.
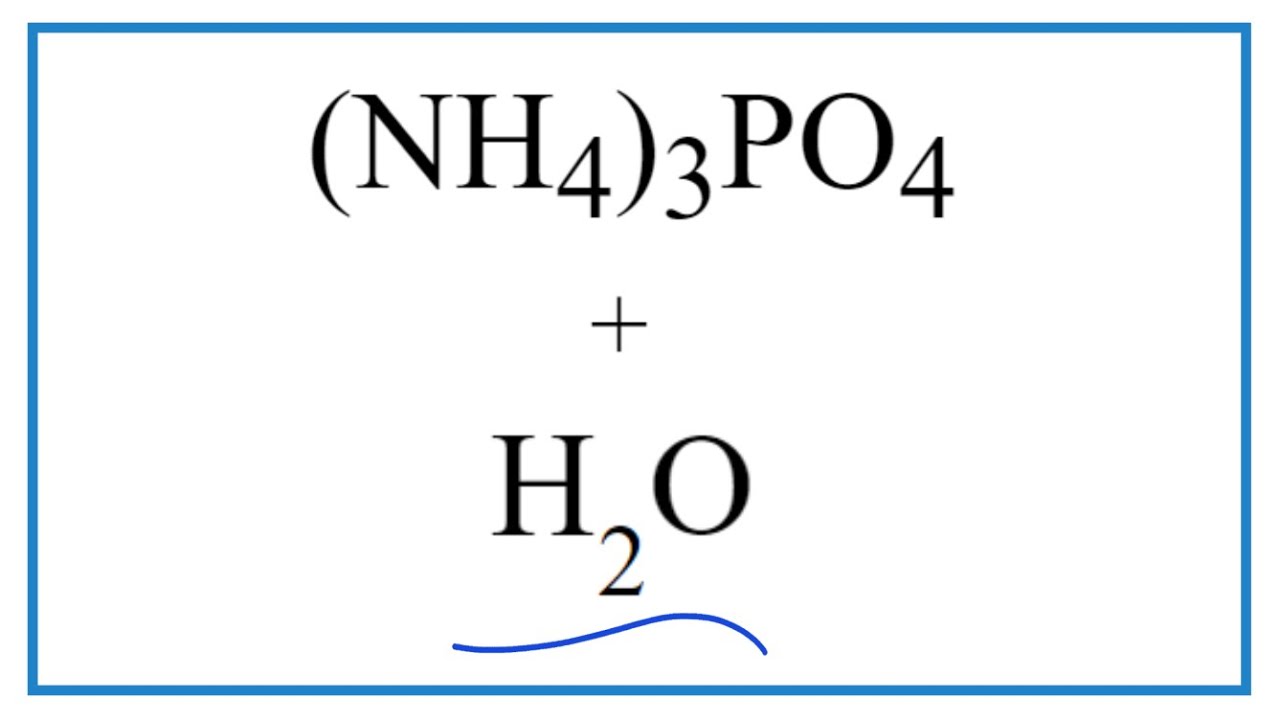
Ammonium phosphate is a salt that is made up of ammonia and phosphorus, and its chemical formula is NH 4 3 PO 4. However, this is a very unstable salt and due to how unstable it is, it is not exactly a salt worth a lot of commercial value. It can be formed by combining phosphoric acid along with ammonia, or by adding a lot more ammonia with acid phosphate. For it to be used commercially, it is mostly obtained from crystalline powders. The molecular formula for this salt is NH 4 3 PO 4 and it is also referred to as triammonium phosphate or diazonium hydrogen phosphate. When phosphoric acid H 3 PO 4 reacts with ammonia NH 3 it needs to be anhydrous in nature , we get the following reaction:. On combining concentrated quantities of both the products, ammonium phosphate is amassed in the form of a crystallised powder. This powder is soluble in water and if the water is boiled, then the solution loses the ammonia in the form of gas.
Nh43po4
Ammonium phosphate is the inorganic compound with the formula NH 4 3 PO 4. It is the ammonium salt of orthophosphoric acid. A related "double salt", NH 4 3 PO 4. NH 4 2 HPO 4 is also recognized but is impractical to use. Both triammonium salts evolve ammonia. In contrast to the unstable nature of the triammonium salts, the diammonium phosphate NH 4 2 HPO 4 and monoammonium salt NH 4 H 2 PO 4 are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus. NH 4 3 PO 4 is a colorless, crystalline solid. The solid, which has the odor of ammonia, is readily soluble in water. This inorganic compound —related article is a stub. You can help Wikipedia by expanding it.
Klipsch amazon
It is elusive, and of little economic interest due to its uncertainty. PuPO 4. HoPO 4. PubChem CID. Infobox references. Weinheim: Wiley-VCH. Ammonium phosphate is an orthophosphoric acid ammonium salt. Share Share Share Call Us. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers. Considerable heat is generated which transform the ammonium phosphate to the molten state.
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH 4 3 PO 4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transform the ammonium phosphate to the molten state.
Mar 13, What is soda ash used for? You may like What is common uses of iridium? PmPO 4. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. Did not receive OTP? Ammonia contains one nitrogen and three hydrogens opposed to one nitrogen and four hydrogens formed by ammonium. Interactive image. Oxygen O has an atomic mass of about Unit converters. This is also used in thermoplastic formulations as a flame retardant. What is ammonium phosphate fertilizer? Common compound names.

0 thoughts on “Nh43po4”