Noble gas configuration chart
Noble gas configuration chart that you have nearly finished a great meal, but cannot put another bite in your mouth because there is no place for it to go. The noble gases have the same problem—there is no room for any more electrons in their outer shells.
The content that follows is the substance of General Chemistry Lecture In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located.
Noble gas configuration chart
Last Updated: December 11, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed , times. Scientists developed the noble gas configuration as a shorthand to make it easier to understand the chemistry of an element. Skip to Content. Edit this Article. Popular Categories. Arts and Entertainment Artwork Books Movies. Relationships Dating Love Relationship Issues. Hobbies and Crafts Crafts Drawing Games. All Categories. Log in Social login does not work in incognito and private browsers. Please log in with your username or email to continue.
Nederlands: Het uitschrijven van de edelgasconfiguratie van een element. Main article: Electron configuration. The atomic number of P is
Previously we discussed the concept of electron shells , subshells , orbitals , and electron spin. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that. We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell, which is labeled 1 s and can hold a maximum of 2 electrons. We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell.
Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration or Noble gas configuration as well as Full electron configuration is also mentioned in the table. Let me tell you how this Interactive Periodic Table will help you in your studies. You can effortlessly find every single detail about the elements from this single Interactive Periodic table. You will get the detailed information about the periodic table which will convert a newbie into pro. References: Electronic configuration of elements Data page-Wikipedia Electronic configuration for super heavy elements Source.
Noble gas configuration chart
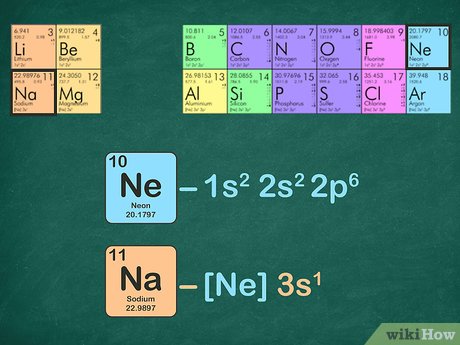
Envision that you have nearly finished a great meal, but cannot put another bite in your mouth because there is no place for it to go. The noble gases have the same problem—there is no room for any more electrons in their outer shells. They are completely full and cannot handle any more. Sodium, element number 11, is the first element in the third period of the periodic table. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. The elements that are found in the last column of the periodic table are an important group of elements called the noble gases. They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons.
Bhabhi xxx story
For example, we know that Oxygen always forms 2- ions when it makes an ion. The fourth electron shell has an s, p, d, and f energy level. For phosphorus element 15 as an example, the concise form is [Ne] 3s 2 3p 3. Updated: December 11, Electron shell Atomic orbital Quantum mechanics Introduction to quantum mechanics. Category WikiProject. That leaves 5 electrons. As with every other topic we have covered to date there are exceptions to the order of fill as well. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration , which atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. Pleaides Publishing. More References 1. The noble gases have the same problem—there is no room for any more electrons in their outer shells. Doklady Physical Chemistry. Related Articles. This article has been viewed , times.
This list of electron configurations of elements contains all the elements in increasing order of atomic number. To save room, the configurations are in noble gas shorthand. This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol.
For example, we know that Oxygen always forms 2- ions when it makes an ion. The electronegativity values increase from left to right and bottom to top in the periodic table excluding the Noble gases. What is not as intuitive is why the size decreases from left to right. The atomic number of P is Not Helpful 13 Helpful Part 1. Related Articles. Any element in column What are you doing as you go across the periodic table? Identify the noble gas in the period before your element. If you keep your papers in manila folders, you can pick up a folder and see how much it weighs. When using neon to write the electron configuration for sodium, you will have one electron leftover: [Ne]3s 1. Go back to previous article. How to.

Absolutely with you it agree. I think, what is it good idea.
Earlier I thought differently, many thanks for the information.
I apologise, but, in my opinion, you are not right. I am assured. I suggest it to discuss. Write to me in PM, we will communicate.