Otf chemistry
With an accout for my. Triflatemore formally known as trifluoromethanesulfonateis a functional group with the formula CF 3 SO 3 - otf chemistry.
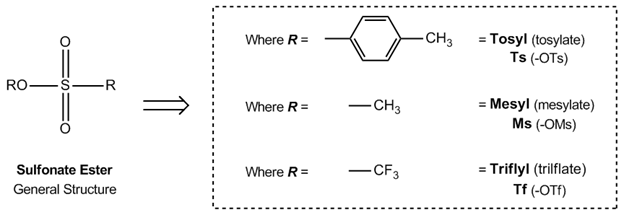
A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution , Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in S N 2 reactions , they must be stored in conditions free of nucleophiles such as water. The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. An additional stabilization is achieved by the trifluoromethyl group, which acts as a strong electron-withdrawing group using the sulfur atom as a bridge. Triflates have also been applied as ligands for group 11 and 13 metals along with lanthanides. Lithium triflates are used in some lithium ion batteries as a component of the electrolyte.
Otf chemistry
.
Lawrance, Peter A.
.
Reaction Map: Reactions of Organometallics. There has been a trend in recent years towards including transition metal catalyzed reactions in the introductory organic chemistry curriculum. The reactions most common covered are palladium catalyzed coupling reactions Suzuki and Heck reactions in particular and olefin metathesis. I generally think this is a bad idea for most courses. More on that at the bottom of the post. However, the fact remains that the material is often covered, and students have to deal with it. Here are three examples. Each of these drugs represents the end result of incredibly expensive multi-year projects by major pharmaceutical companies and have generated untold billions of dollars in revenue. I merely want to make the point here that organic chemists involved in this endeavour:.
Otf chemistry
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Covalent organic frameworks, cross-linked crystalline polymers constructed from rigid organic precursors connected by covalent interactions, have emerged as a promising class of nanoporous materials owing to their highly desirable combination of attributes, including facile chemical tunability, structural diversity, and excellent stability. Despite the distinct advantages offered by three-dimensional covalent organic frameworks, research efforts have predominantly focused on the more synthetically-accessible, two-dimensional variants. Here we present an overview of synthetic approaches to yield three-dimensional covalent organic frameworks, identify synthetic obstacles that have hindered progress in the field and recently-employed methods to address them, and propose alternative techniques to circumvent these synthetic challenges. Nanoporous materials have garnered tremendous interest in recent years owing to their specific and exceptional attributes, notably permanent porosity and large and accessible internal surface areas 1 , 2 , 3 , 4 , 5.
Lowes fire detectors
My watch list My saved searches My saved topics My newsletter Register free of charge. In other projects. Triflates are used as Lewis acids in organic chemistry because of their stability compared to more traditional catalysts unstable in water such as aluminum chloride. Lawrance, Peter A. Journal of the American Chemical Society. Keep logged in. With an accout for my. European Journal of Organic Chemistry. Cookies deactivated. A list of authors is available in Wikipedia.
Trifluoromethanesulfonic anhydride , also known as triflic anhydride, is the chemical compound with the formula CF 3 SO 2 2 O.
Additional recommended knowledge Better weighing performance in 6 easy steps Recognize and detect the effects of electrostatic charges on your balance Daily Visual Balance Check Contents 1 Applications 2 Triflate salts 3 See also 4 References. Your browser is not current. DE please activate JavaScript. The anion owes its stability to resonance stabilization which causes the negative charge to be spread over the three oxygen atoms and the sulfur atom. Triflates are used as Lewis acids in organic chemistry because of their stability compared to more traditional catalysts unstable in water such as aluminum chloride. The new intuitive software for your spectra search. Tools Tools. To top. Keep logged in. Lawrance, Peter A. Download as PDF Printable version. Wikimedia Commons. PMID

In my opinion you have deceived, as child.
It not a joke!
What nice idea