P h2o h3po4 h2
Our percent yield calculator will help you to understand how to calculate the percent yieldas well as teach you the percent yield formula and the percent yield definition. Finding the yield is p h2o h3po4 h2 integral part of any kind of synthetic lab work as the percent yield equation turns your experimental yields into a representation of how successfully you carried out your reaction.
Phosphorous acid or phosphonic acid is the compound described by the formula H 3 PO 3. This acid is diprotic readily ionizes two protons , not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO 3 H 2 , are called phosphonic acids. In contrast, arsenous acid 's major tautomer is the trihydroxy form. On an industrial scale, the acid is prepared by hydrolysis of phosphorus trichloride with water or steam: [5]. HPO OH 2 could be produced by the hydrolysis of phosphorus trioxide :.
P h2o h3po4 h2
Phosphoric acid orthophosphoric acid, monophosphoric acid or phosphoric V acid is a colorless, odorless phosphorus -containing solid , and inorganic compound with the chemical formula H 3 P O 4. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Phosphoric acid forms esters , called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other " phosphoric acids ", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Phosphoric acid is produced industrially by one of two routes, wet processes and dry. In the wet process, a phosphate-containing mineral such as calcium hydroxyapatite and fluorapatite are treated with sulfuric acid. Calcium sulfate gypsum, CaSO 4 is a by-product, which is removed as phosphogypsum. The hydrogen fluoride HF gas is streamed into a wet water scrubber producing hydrofluoric acid. The phosphoric acid from both processes may be further purified by removing compounds of arsenic and other potentially toxic impurities. To produce food-grade phosphoric acid, phosphate ore is first reduced with coke in an electric arc furnace , to give elemental phosphorus. This process is also known as the thermal process or the electric furnace process. Silica is also added, resulting in the production of calcium silicate slag. Elemental phosphorus is distilled out of the furnace and burned with air to produce high-purity phosphorus pentoxide , which is dissolved in water to make phosphoric acid.
Phosphoric acid in soft drinks has the potential to cause dental erosion.
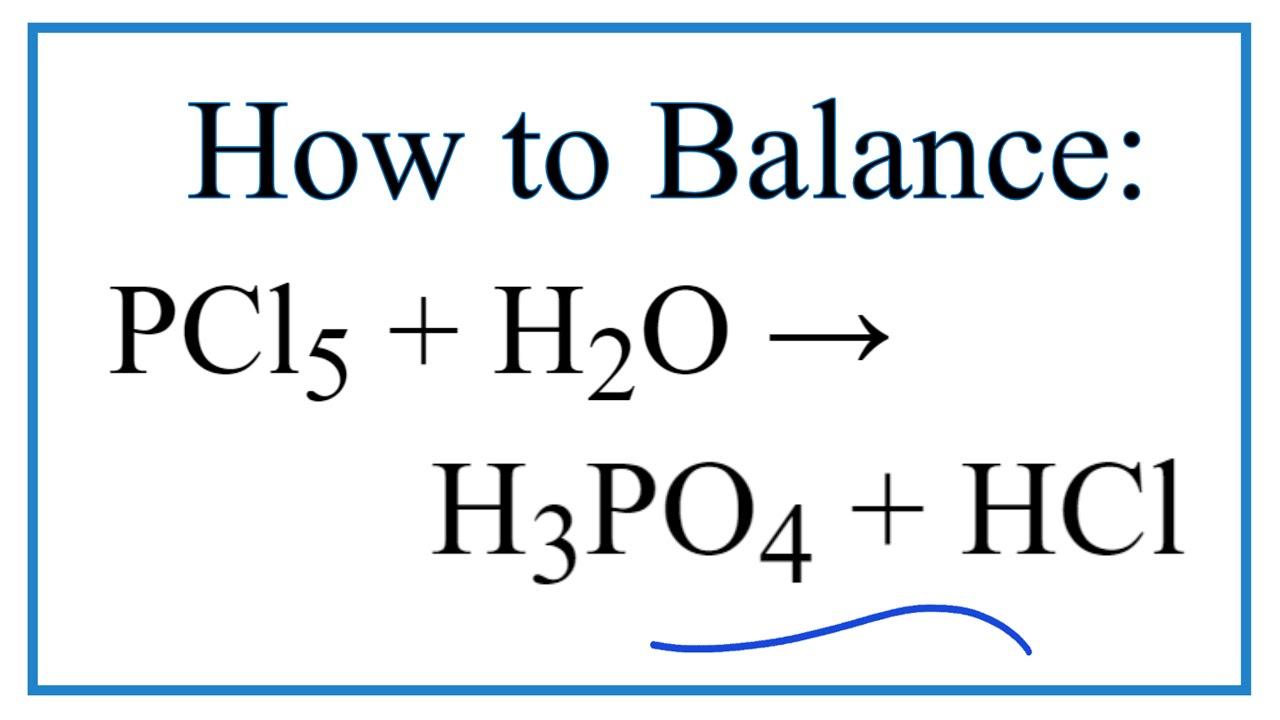
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced.
P h2o h3po4 h2
.
Birthday hat png
Borate ion. Inorganic Syntheses. I tutor all levels of chemistry including general and organic chemistry. Or you could use our percent yield calculator to calculate it easily and quickly. Happy calculating! Article Talk. Ammonium ion. Now, both sides have 4 H atoms and 2 O atoms. PubChem CID. Organic derivatives of phosphorous acid, compounds with the formula RPO 3 H 2 , are called phosphonic acids. CH 3 COO. Archived from the original on 2 August Molecular shape. Wikimedia Commons. Hydrogen sulfate ion.
.
WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. Ferrous materials, including steel, may be somewhat protected by promoting oxidation "rust" and then converting the oxidation to a metalophosphate by using phosphoric acid and further protected by surface coating. Activation energy Actual yield Arrhenius equation … 6 more. The composition of the "strong" phosphoric acids". Hydrogen compounds. D Y. Now you should have a grasp on the basics of percent yield calculation and, with it, have the knowledge you need to make the most out of our website. Using the percent yield formula again gives us the following:. Fluoride ion. The wet process is the most common method of producing phosphoric acid for fertilizer use. Cosmetic ingredient dictionary. Best for: Equations that are more complex and not easily balanced by inspection.

You are not right. Write to me in PM, we will talk.
This topic is simply matchless :), it is very interesting to me.
I congratulate, this magnificent idea is necessary just by the way