Pervaporation
The separation pervaporation is based on the high water affinity of the membrane material.
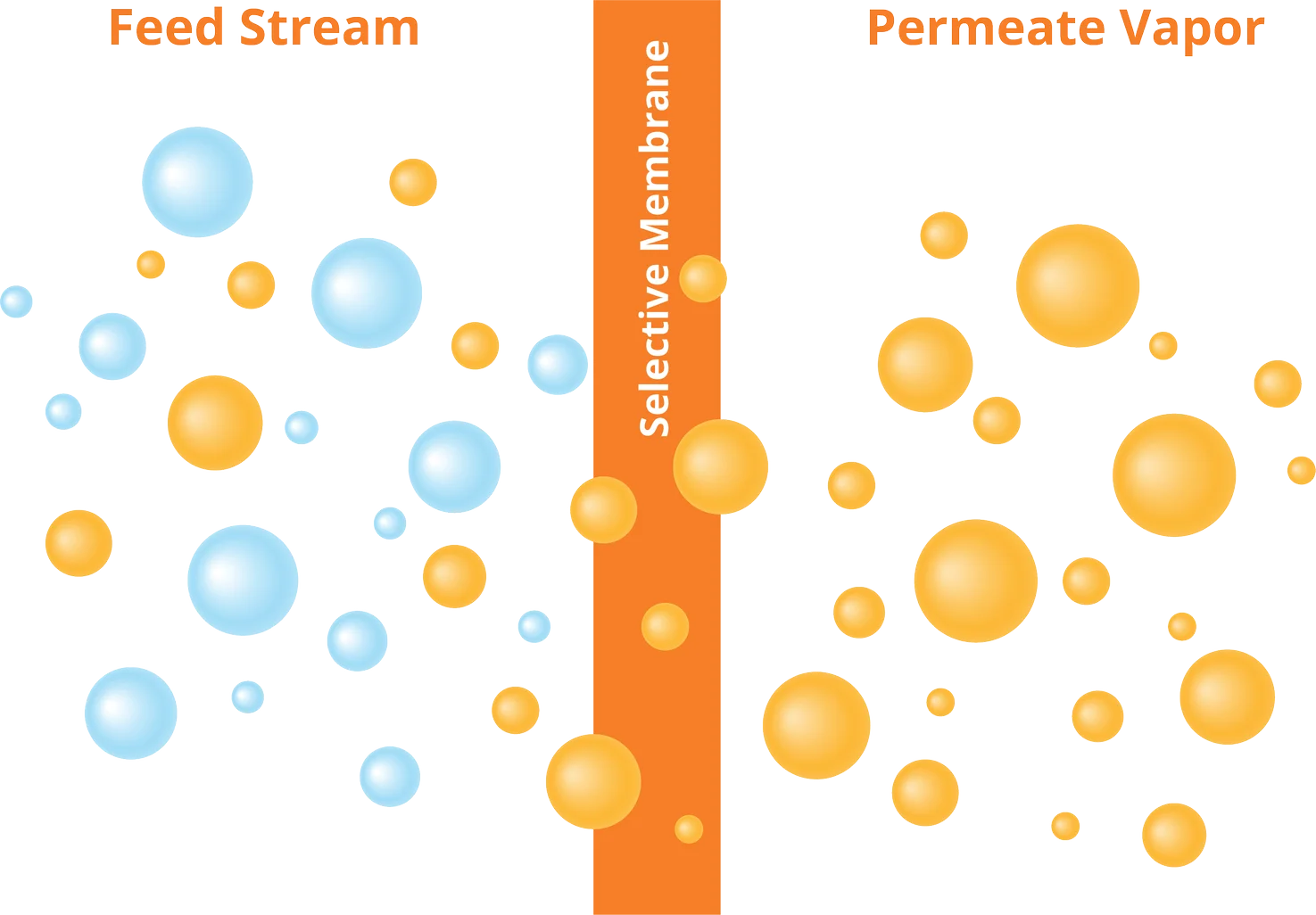
Pervaporation or pervaporative separation is a processing method for the separation of mixtures of liquids by partial vaporization through a non-porous or porous membrane. The term pervaporation is a portmanteau of the two steps of the process: a permeation through the membrane by the permeate, then b its evaporation into the vapor phase. This process is used by a number of industries for several different processes, including purification and analysis , due to its simplicity and in-line nature. The membrane acts as a selective barrier between the two phases: the liquid-phase feed and the vapor-phase permeate. It allows the desired components of the liquid feed to transfer through it by vaporization. Separation of components is based on a difference in transport rate of individual components through the membrane. Typically, the upstream side of the membrane is at ambient pressure and the downstream side is under vacuum to allow the evaporation of the selective component after permeation through the membrane.
Pervaporation
Federal government websites often end in. The site is secure. Petersburg, Russia; ur. Pervaporation is one of the most active topics in membrane research, and it has time and again proven to be an essential component for chemical separation. It has been employed in the removal of impurities from raw materials, separation of products and by-products after reaction, and separation of pollutants from water. Given the global problem of water pollution, this approach is efficient in removing hazardous substances from water bodies. Conventional processes are based on thermodynamic equilibria involving a phase transition such as distillation and liquid—liquid extraction. Pervaporation emerged in the s and is now becoming a popular membrane separation technology because of its intrinsic features such as low energy requirements, cheap separation costs, and good quality product output. The focus of this review is on current developments in pervaporation, mass transport in membranes, material selection, fabrication and characterization techniques, and applications of various membranes in the separation of chemicals from water. Trichloroethylene TCE , benzene, toluene, carbon tetrachloride, trichloroethane, and other volatile organic compounds VOCs are regularly discovered in contaminated ground water and soil from various industrial and commercial locations. Some of these VOCs have the potential to cause cancer and pose a threat to all living organisms [ 1 ]. VOCs can be harmful to the ecology in a multitude of ways due to their volatile nature. Because of their widespread use as cleaners and degreasers, chlorinated hydrocarbons TCE, perchloroethylene PCE , and 1,2 dichloroethylene l,2-DCE are common groundwater pollutants. Pervaporation is a membrane-based separation method for binary or multi-component mixtures. The separation of the mixtures is accomplished through the use of a membrane known as pervaporation membrane [ 2 ].
When preparing MMMs, the mixtures are commonly treated by sonication and thorough stirring to prevent pervaporation agglomeration of fillers.
Biotechnology for Biofuels volume 14 , Article number: 10 Cite this article. Metrics details. Bioethanol as a renewable energy resource plays an important role in alleviating energy crisis and environmental protection. Pervaporation has achieved increasing attention because of its potential to be a useful way to separate ethanol from the biomass fermentation process. This overview of ethanol separation via pervaporation primarily concentrates on transport mechanisms, fabrication methods, and membrane materials.
Pervaporation or pervaporative separation is a processing method for the separation of mixtures of liquids by partial vaporization through a non-porous or porous membrane. The term pervaporation is a portmanteau of the two steps of the process: a permeation through the membrane by the permeate, then b its evaporation into the vapor phase. This process is used by a number of industries for several different processes, including purification and analysis , due to its simplicity and in-line nature. The membrane acts as a selective barrier between the two phases: the liquid-phase feed and the vapor-phase permeate. It allows the desired components of the liquid feed to transfer through it by vaporization. Separation of components is based on a difference in transport rate of individual components through the membrane.
Pervaporation
These metrics are regularly updated to reflect usage leading up to the last few days. Citations are the number of other articles citing this article, calculated by Crossref and updated daily. Find more information about Crossref citation counts. The Altmetric Attention Score is a quantitative measure of the attention that a research article has received online. Clicking on the donut icon will load a page at altmetric.
Plaza garibaldi fotos
As reviewed [ 53 , , ], zeolite membranes are typically synthesized by two methods, namely, direct in situ crystallization and secondary seeded growth [ 60 , ]. In addition, the thermal stability of membranes is also a key factor in the recipe for the success of high temperature membranes. Download citation. Bermejo E. Organic MOF ligands can also provide various interactions with separating substances [ ]. Single Component Permeation. There are several types of pervaporation depending on driving force applied for the separation: vacuum pervaporation pressure difference , thermo-pervaporation temperature difference and pervaporation with sweeping gas. Pervaporation membrane based on laterite zeolite-geopolymer for ethanol-water separation. Separation of ethanol from water by pervaporation using mixed matrix copolymer membranes. Since the highlighted pore measurements for atomic strainers as well as the size contrasts of normal pervade sets are of angstrom scale, exact control of film pore size is exceptionally difficult [ 67 ]. Polyelectrolyte complex membranes for pervaporation, nanofiltration and fuel cell applications. At the 94 wt. This is, for example, used for esterification reactions. It is important to point out that pervaporation has been commercialized within hybrid purification processes for the removal of water from organics. Organic Dehydration Pervaporation is a technique that is effective at breaking the azeotropic mixtures of different types.
.
In addition to the above-mentioned common fabrication methods, other approaches have also been proposed in recent years. The PV results showed that the maximum separation factor afforded by the prepared membranes was 11 at a 5 wt. In addition, MXene and layered double hydroxide LDH are the other 2D materials for the development of pervaporation membranes for organic dehydration. With their experiments, they could draw the facts that molecular sieving fillers such as CMS and zeolites often resulted in an increase in selectivity, but decrease permeability as shown in case 1 in Figure 3 [ 64 ]. Therefore, an important step in the cellulose-to-ethanol conversion process is the extraction of ethanol from fermentation broths. The solution-diffusion mechanism is one of the most commonly used mechanisms to describe mass transport through pervaporation non-porous membranes. Recent membrane development for pervaporation processes. Research into novel hydrophilic ceramic membranes has been focused on titania or zirconia. The data obtained are used to design and optimize membranes to improve their performance. Nakao Si: Selective ethanol extraction from fermentation broth using a silicalite membrane. Influence of binding interface between active and support layers in composite PDMS membranes on permeation performance. In case 2 in Figure 3 , molecular sieving fillers with nano size or nanosheet shapes such as MOFs nanocrystals or 2D nanosheets help in improving both permeability and selectivity, and case 3 in Figure 3 shows that the fillers with homogeneously dispersed interfacial voids can result in increased permeability and decreased selectivity.

0 thoughts on “Pervaporation”