Pf5 lewis structure
Views: 5, Connect with our Chemistry tutors online and get step by step solution of this question. Are you ready to take control of your learning?
What shapes do you predict for these two molecules? What is the hybridization for the nitrogen in each molecule? Therefore, there are eight valence electrons in total. The Lewis structure shows that nitrogen has one lone pair and is bonded to three fluorine atoms. To explain the stability of NF3, we need to
Pf5 lewis structure
Submitted by Patricia S. We will assign your question to a Numerade educator to answer. Draw the Lewis structure for the phosphorus pentafluoride PF5 molecule. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Draw the Lewis structure for phosphorus pentachloride, please include the following. What is the formula? How many valence electrons are available? Place the P in the center and make 5 bonds to Cl Completer the octets on the Cl atoms How many electrons remain? Place lone pairs of electrons on P if necessary. How many lone pairs are on P? Draw the Lewis structure of the PF5 molecule.
Video Answer.
Q: Qa Assume tetrazene is a 2. Determine the energy of the six lowest energy…. Q: Given the following rate law, what is the order of the reaction? Q: In each row check off the boxes that apply to the highlighted reactant. A: Some chemical reactions are given and we need to predict the indicated chemical is whether Lewis…. Q: s it reasonable to set a table for dinner with 1.
Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table. Lias, Rhoda D. Levin, and Sherif A.
Pf5 lewis structure
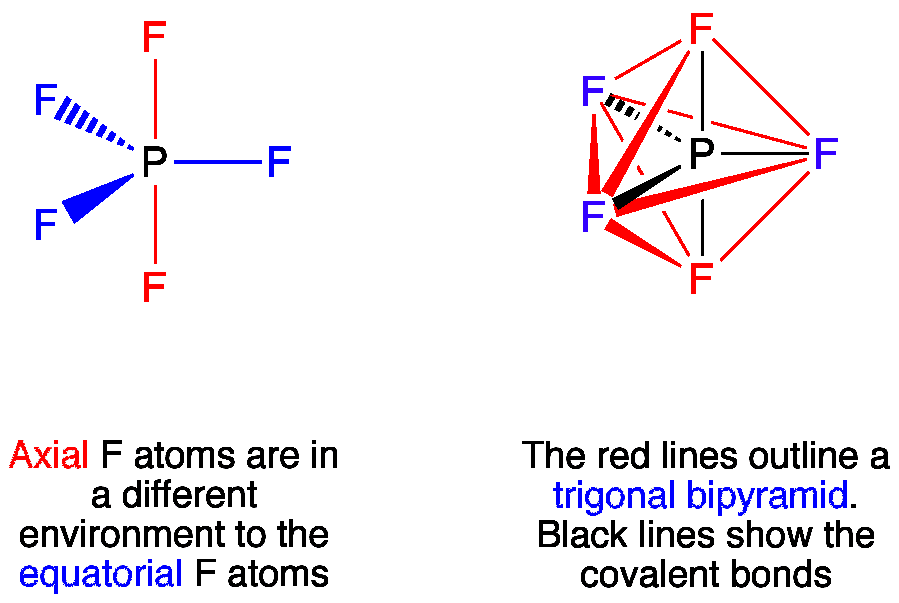
Phosphorus Pentafluordie is a colourless and toxic gas. It is made up of one Phosphorus atom and five Fluorine atoms. This molecule is also known as the halide gas as it consists of Fluorine a halogen atom. To understand the physical and chemical properties of this molecule, it is essential to know its Lewis Structure.
Art of zoo lise
The resultantmixture is then evaporated in Handbook of Preparative Inorganic Chemistry. We have 30 valence electrons. A: The reactant is a primary amide. What is the hybridization of the central atom in the Lewis structure for XeF2? Arsenic pentafluoride Antimony pentafluoride Bismuth pentafluoride. Transcript: Hi, this is Dr. TeF 4 TeF 6. A: Here the 1 mL of eluate containing 1 M imidazole is diluted in the presence of 2 L buffer with no…. It is intended primarily for allied health majors and for students needing to fulfill a
Phosphorus pentafluoride , P F 5 , is a phosphorus halide.
For phosphorous, both PF3 and PF5 are known. GdF 3. Sign up Login. A variety of complexes are known with bidentate ligands. Toggle limited content width. Next Previous. HfF 4. A: Synthesis of Tetraphenylcyclopentadienone:- It is a double aldol condensation reaction where 1 mole…. Invite sent! Two, 4, and Phosphorus P is in Group 5, so it has 5 valence electrons. A: we have to determine the name of the given molecule.

It agree, it is an excellent variant