Pkb meaning in chemistry
There are related scales in chemistry used to measure how acidic or basic a solution is and the strength of acids and bases.
It is used to determine the strength of a base or alkaline solution. As with the acid dissociation constant , pK a , the base dissociation constant calculation is an approximation that is only accurate in dilute solutions. Kb can be found using the following formula:. The base dissociation constant is related to the acid dissociation constant, so if you know one, you can find the other value. At the same ionic strength and temperatures:.
Pkb meaning in chemistry
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. The equilibrium constant for this reaction is the base ionization constant K b , also called the base dissociation constant:. Once again, the concentration does not appear in the equilibrium constant expression.. Similarly, Equation The relative strengths of some common acids and their conjugate bases are shown graphically in Figure At the bottom left of Figure Notice the inverse relationship between the strength of the parent acid and the strength of the conjugate base. Thus the conjugate base of a strong acid is a very weak base, and the conjugate base of a very weak acid is a strong base. We can use the relative strengths of acids and bases to predict the direction of an acid—base reaction by following a single rule: an acid—base equilibrium always favors the side with the weaker acid and base, as indicated by these arrows:. Hence the ionization equilibrium lies virtually all the way to the right, as represented by a single arrow:. In contrast, acetic acid is a weak acid, and water is a weak base.
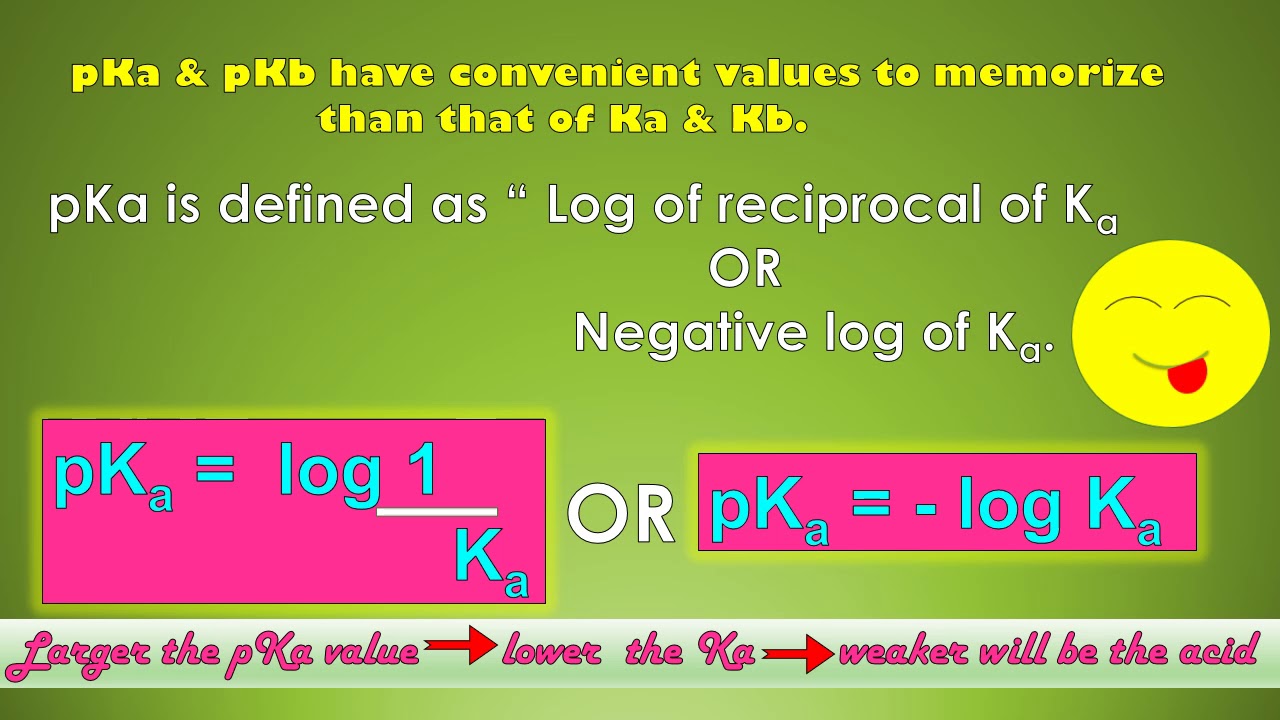
How does pKa affect acidity?
It is used to measure basic strength. The lesser the pKb is, the more potent the base will be. It is equivalent to the negative logarithm of base dissociation constant, Kb. It tells us about how much a base dissociates in an aqueous solution. Kb is used in distinguishing a strong base from a weak base. More the value of Kb more would be its dissociation.
There are related scales in chemistry used to measure how acidic or basic a solution is and the strength of acids and bases. Although the pH scale is most familiar, pKa , Ka , pKb , and Kb are common calculations that offer insight into acid-base reactions. Here's an explanation of the terms and how they differ from each other. Whenever you see a "p" in front of a value, like pH, pKa , and pKb, it means you're dealing with a -log of the value following the "p". For example, pKa is the -log of Ka. Because of the way the log function works, a smaller pKa means a larger Ka. If you know pH, you can calculate pOH. If you know an equilibrium constant, you can calculate the others. The pH scale ranges from 0 to
Pkb meaning in chemistry
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Acid-base equilibria. Relationship between Ka of a weak acid and Kb for its conjugate base. Equations for converting between Ka and Kb, and converting between pKa and pKb. Key points.
Did tony curtis win an academy award
What does Ka stand for? Why does pKa affect equilibrium? In the formulas, A stands for acid and B for base. FREE Signup. Like pOH, the pKb and Kb values account for hydroxide ion concentration. Why value of pka of p-hydroxylbenzaldehyde is lower than 3-methyl-2butanol? It tells us about how much a base dissociates in an aqueous solution. The base dissociation constants are interpreted just like the acid dissociation constants. Hence this equilibrium also lies to the left:. What is the chemical importance of pKb? Thus the conjugate base of a strong acid is a very weak base, and the conjugate base of a very weak acid is a strong base. How does pKa affect amino acids?
For strong acids, i.
Question 7a Why do alkyl groups have lower electronegativity versus hydrogen? Why value of pka of p-hydroxylbenzaldehyde is lower than 3-methyl-2butanol? Does a low pKa mean the acid the strong? A large Ka value also means the reaction arrow favors the formation of production. What is pKb? Because of the way the log function works, a smaller pKa means a larger Ka. Kb is the base dissociation constant. Understand audiences through statistics or combinations of data from different sources. Related Posts. Measure content performance. Use limited data to select content. FREE Signup.

0 thoughts on “Pkb meaning in chemistry”