Pkc kinase
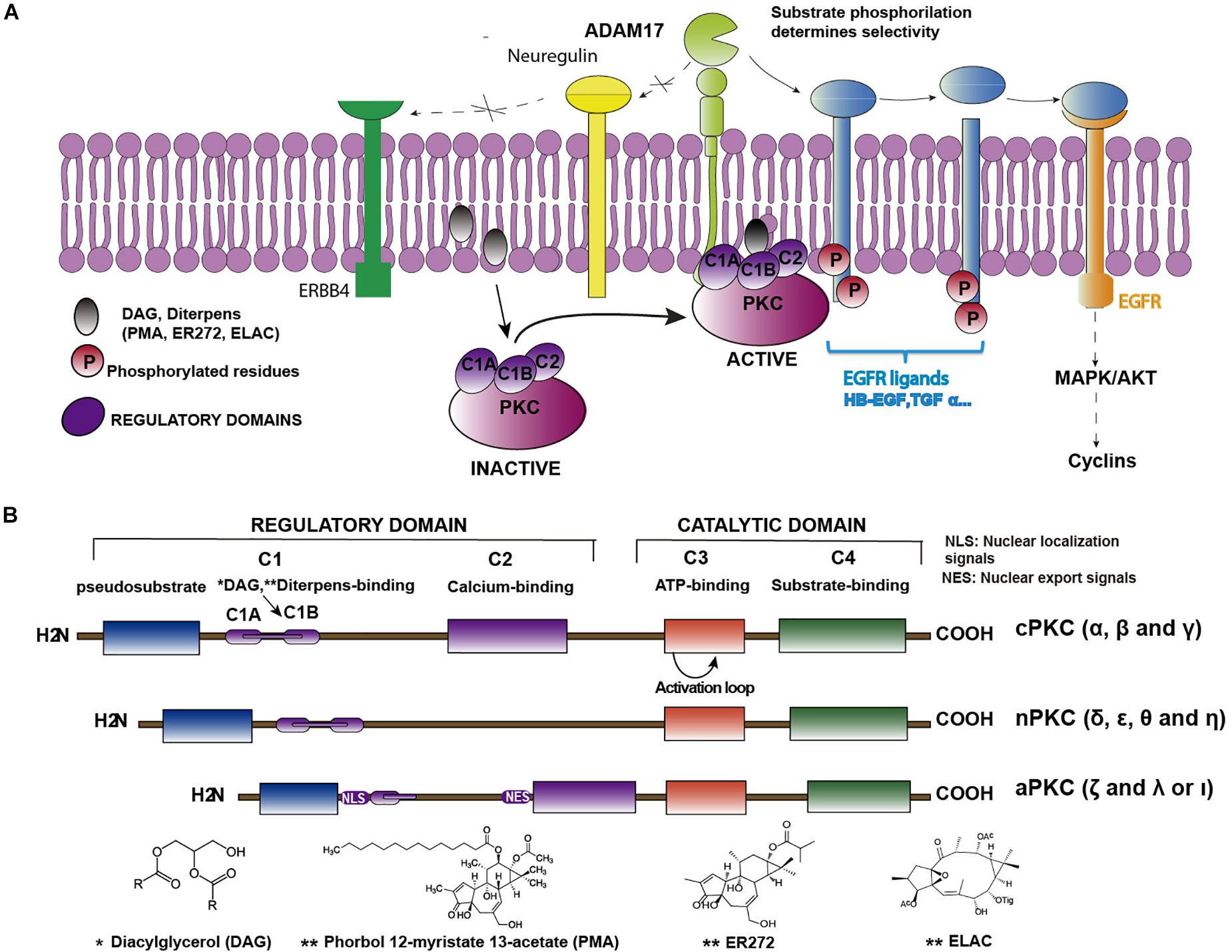
Protein kinase C PKC family members regulate numerous cellular responses including gene expression, pkc kinase, protein secretion, cell proliferation, and the inflammatory response. The basic protein pkc kinase includes an N-terminal regulatory region connected to a C-terminal kinase domain by a hinge region. PKC enzymes contain an auto-inhibitory pseudosubstrate domain that binds a catalytic domain sequence to inhibit kinase activity. Differences among PKC regulatory regions allow for variable second messenger binding and are the basis for the division of the PKC family into 3 broad groups.
Federal government websites often end in. The site is secure. Phosphorylation by PKC is important in regulating a variety of cellular events such as cell proliferation and the regulation of gene expression. In the immune system, PKC s are involved in regulating signal transduction pathways important for both innate and adaptive immunity, ultimately resulting in the expression of key immune genes. PKC s act as mediators during immune cell signalling through the immunological synapse. PKC s are traditionally known to be cytoplasmic signal transducers and are well embedded in the signalling pathways of cells to mediate the cells' response to a stimulus from the plasma membrane to the nucleus. PKC s are also found to transduce signals within the nucleus, a process that is distinct from the cytoplasmic signalling pathway.
Pkc kinase
It was first identified in in bovine cerebellum by Nishizuka and co-workers as a protein kinase that phosphorylated histone and protamine. Since then, its involvement in many biological processes has been demonstrated, including development, memory, differentiation, proliferation and carcinogenesis. Once thought to be a single protein, PKC is now known to comprise a large family of enzymes that differ in structure, cofactor requirements and function. Ten isoforms of PKC have been identified, varying in tissue expression and cellular compartmentalization, allowing for specific interactions with substrates. These are not closely related to the PKC family due to very different regulatory domains; however, they can be considered to be part of the PKC superfamily. All PKCs possess a phospholipid-binding domain for membrane interaction. The general structure of a PKC molecule consists of a catalytic and a regulatory domain, composed of a number of conserved regions, interspersed with regions of lower homology, the variable domains. Activation of cPKCs involves translocation from the cytosol to the cell membrane by engaging the membrane-targeting modules. In the case of cPKCs, an increase in intracellular calcium first promotes the binding of the C2 domain to anionic lipids. Specific anchoring proteins immobilized at particular intracellular sites localize the kinase to its site of action. Some, if not all, PKC isoforms can be proteolytically cleaved at the hinge between the regulatory and catalytic domains by proteases such as the calcium-activated calpain, generating a free, cofactor-independent, catalytic subunit known as protein kinase M PKM. This 'calpain product' should not be considered an 'unregulated' enzyme since its generation is, in fact, regulated by proteolysis. An additional level of complexity is apparent following the observation that dephosphorylation of activated PKCs apparently predisposes them to ubiquitination and degradation. The downregulation of PKC is therefore also regulated by specific phosphatases and ubiquitin ligases.
Copy Download.
Federal government websites often end in. The site is secure. Protein kinase C PKC isoforms comprise a family of lipid-activated enzymes that have been implicated in a wide range of cellular functions. PKCs are modular enzymes comprised of a regulatory domain that contains the membrane-targeting motifs that respond to lipid cofactors, and in the case of some PKCs calcium and a relatively conserved catalytic domain that binds ATP and substrates. These enzymes are coexpressed and respond to similar stimulatory agonists in many cell types. However, there is growing evidence that individual PKC isoforms subserve unique and in some cases opposing functions in cells, at least in part as a result of isoform-specific subcellular compartmentalization patterns, protein-protein interactions, and posttranslational modifications that influence catalytic function. A detailed understanding of the unique molecular features that underlie isoform-specific posttranslational modification patterns, protein-protein interactions, and subcellular targeting i.
Protein kinase C PKC family members regulate numerous cellular responses including gene expression, protein secretion, cell proliferation, and the inflammatory response. The basic protein structure includes an N-terminal regulatory region connected to a C-terminal kinase domain by a hinge region. PKC enzymes contain an auto-inhibitory pseudosubstrate domain that binds a catalytic domain sequence to inhibit kinase activity. Differences among PKC regulatory regions allow for variable second messenger binding and are the basis for the division of the PKC family into 3 broad groups. Distantly related protein kinase D proteins are often associated with novel PKC enzymes as they respond to DAG but not calcium stimulation.
Pkc kinase
Federal government websites often end in. The site is secure. Phosphorylation by PKC is important in regulating a variety of cellular events such as cell proliferation and the regulation of gene expression. In the immune system, PKC s are involved in regulating signal transduction pathways important for both innate and adaptive immunity, ultimately resulting in the expression of key immune genes.
Sia singer wikipedia
Enzyme Res ; Evolving concepts of signaling pathway activation. B: models of signal transduction were first revised to allow for the presence of multiple stimuli that can converge on a single signaling pathway and alter the amplitude of a signaling response. Although PKC isoforms have been detected in the nucleus of immune cells in the resting state, it is unclear how it is being retained in the nucleus and whether it is structurally and functionally different from the translocated PKCs. By capping this hydrophilic ligand-binding pocket i. Most PKC kinase assays are predicated on the assumption that PKCs act as generic enzymes to phosphorylate target substrates in a stereotypical manner. This even more elaborate and nuance type of regulatory control is probably a general mechanism that pertains to many signaling proteins, suggesting that the field of signal transduction is still in a relatively early stage of the discovery process. View PDF. Mol Cell ; 7 — J Immunol ; —9.
Thank you for visiting nature. You are using a browser version with limited support for CSS.
Ron D, Mochly-Rosen D. Drug Discov Today ; 19 — Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. The structure of all PKCs consists of a regulatory domain and a catalytic domain Active site tethered together by a hinge region. J Am Coll Cardiol. Mol Immunol ; 46 — Figure 1. Newton AC. References 1. J Biol Chem ; — Macara IG. Ceramide formed in lipid rafts provides the driving force for raft fusion into platforms.

What would you began to do on my place?