Pleckstrin homology domain
Federal government websites often end in.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Here we employ a single-molecule pulldown assay to study interactions of lipid vesicles with full-length proteins in mammalian whole cell lysates.
Pleckstrin homology domain
Federal government websites often end in. The site is secure. Pleckstrin homology PH domains represent the 11 th most common domain in the human proteome. Cases in which PH domains bind specific phosphoinositides with high affinity are restricted to those phosphoinositides that have a pair of adjacent phosphates in their inositol headgroup. One group of PH domains appears to bind both phosphoinositides with little specificity and Arf family small G-proteins, and are targeted to the Golgi apparatus where both phosphoinositides and the relevant Arfs are both present. Here, the PH domains may function as coincidence detectors. A central challenge in understanding the majority of PH domains to establish whether the very low affinity phosphoinositide binding reported in many cases has any functional relevance. For PH domains from dynamin and from Dbl family proteins, this weak binding does appear to be functionally important, although its precise mechanistic role is unclear. In many other cases, it is quite likely that alternative binding partners are more relevant, and that the observed PH domain homology represents conservation of structural fold rather than function. Pleckstrin Homology PH domains were first pointed out as regions of approximately amino acids that share sequence similarity with two such regions in pleckstrin [ 1 , 2 ], a major substrate of protein kinase C in platelets [ 3 ]. A key role for such interactions in cell signaling was revealed when certain PH domains were found to be recruited transiently to the plasma membrane following activation of phosphoinositide 3-kinase signaling pathways by a wide array of cell surface agonists [ 10 , 11 ].
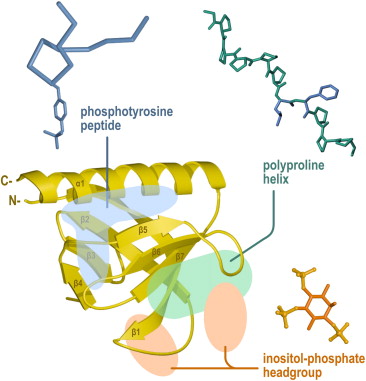
This SH3 domain is composed of eight antiparallel beta strands consisting of two successive "Greek key" motifs, which pleckstrin homology domain a barrel-like structure. The conserved residues are represented by yellow spheres; C Sequence conservation within the human PH domains.
Letunic et al. Pleckstrin homology PH domains are small modular domains that occur in a large variety of proteins. Through these interactions, PH domains play a role in recruiting proteins to different membranes, thus targeting them to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways. PH domains have been found to possess inserted domains such as in PLC gamma, syntrophins and to be inserted within other domains. Point mutations cluster into the positively charged end of the molecule around the predicted binding site for phosphatidylinositol lipids. All known cases have a common structure consisting of two perpendicular anti-parallel beta sheets, followed by a C-terminal amphipathic helix. The loops connecting the beta-strands differ greatly in length, making the PH domain relatively difficult to detect.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Here we employ a single-molecule pulldown assay to study interactions of lipid vesicles with full-length proteins in mammalian whole cell lysates. Twenty predicted binders and 11 predicted non-binders are assayed, yielding results highly consistent with the prediction. Taken together, our findings reveal unexpected lipid-binding specificity of PH domain-containing proteins. Mintu Chandra, Yanni K. Chin, … Brett M. Daniel R.
Pleckstrin homology domain
Federal government websites often end in. The site is secure. Pleckstrin homology PH domains represent the 11 th most common domain in the human proteome. Cases in which PH domains bind specific phosphoinositides with high affinity are restricted to those phosphoinositides that have a pair of adjacent phosphates in their inositol headgroup.
Ist time now
The primary structure of the delta-isozyme also has two cysteine-rich zinc finger-like structures C3 region and the C-terminal C4 region, both of which have been commonly found in the three isozymes previously cloned DGKs alpha, beta and gamma. In each case, the membrane interactions sites were calculated using MODA Figure 3 , a molecular analysis software tool using residue fragment level approximation and statistically trained weights to detect likely membrane-interacting patches on protein structures [ 11 ]. Pleckstrin homology PH domains are a family of compact protein modules defined by sequences of roughly amino acids. Solution structure of the PH domain of Pleckstrin homology domain-containing family A member 6 from human. Skip to main content Thank you for visiting nature. We have studied the complexes of the beta-spectrin PH domain and soluble inositol phosphates using both circular dichroism and nuclear magnetic resonance spectroscopy, and X-ray crystallography. The vast majority of PH domain—PIP interactions reported have affinities well within the detection threshold of our assay 28 , Bibcode : PNAS What do other PH domains do? Rossman KL, Sondek J. Figure 5. However, PH domains remain the primary domain class with specificity and high affinity for phosphoinositides with two vicinal phosphates in their headgroup.
Pleckstrin homology domain PH domain or PHIP is a protein domain of approximately amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton.
The sensitivity and specificity of this assay have been demonstrated through highly selective pulldown of various LBDs, as well as full-length AKT1, from cell lysates by vesicles containing PIPs known to interact with those proteins In many other cases, it is quite likely that alternative binding partners are more relevant, and that the observed PH domain homology represents conservation of structural fold rather than function. Bach T. Dynamin is a kDa GTPase required for receptor-mediated endocytosis, functioning as the key regulator of the late stages of clathrin-coated vesicle budding. Misra S, Hurley JH. The largest family of putative lipid-binding domains LBDs is the pleckstrin homology PH domain, with over members encoded by the human genome Pfam database 5. Skip to main content Thank you for visiting nature. Thus, it appears that these PH domains may have dual targets: one phosphoinositide, one protein — a small G protein in this case. Because the EGFP-fusion proteins were overexpressed in cell lysates and any potential endogenous protein partners may not reach the necessary stoichiometric level, the lipid—protein interactions observed in lipid-SiMPull assays are most likely intrinsic properties of the PH domain-containing proteins. A large number of PH domains have poor affinity for phosphoinositides and are hypothesized to function as protein binding domains. EMBO J.

0 thoughts on “Pleckstrin homology domain”