Propanone dot structure
Dont't have an account? Register Now. Colleges Colleges Accepting B.
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. In all cases, the same types of diagrams are used to indicate where electrons and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. An octet rule governs Lewis structures. Lewis structures are useful for understanding chemical bonding. However, they lack the ability to account for aromaticity and do not accurately mimic magnetic behaviour.
Propanone dot structure
.
Who do you change sugarcane as black colour turn to white Who they will change the colour of sugarcane black to white.
.
The carbonyl group is ubiquitous in biological compounds. It is found in carbohydrates, fats, proteins, nucleic acids, hormones, and vitamins—organic compounds critical to living systems. In a ketone, two carbon groups are attached to the carbonyl carbon atom. The following general formulas, in which R represents an alkyl group and Ar stands for an aryl group, represent ketones. In an aldehyde, at least one of the attached groups must be a hydrogen atom. The following compounds are aldehydes:.
Propanone dot structure
The chemical formula C 3 H 6 O represents acetone. This compound is also referred to as propanone or, in some texts as Propanone. Acetone is considered to be the simplest form of Ketone. This compound is colorless, flammable, and is pungent smelling. Acetone is an important organic solvent, and it is used in the dissolution of fats, resins, and many other organic substances. It is also readily miscible in water. Acetone is widely used in various applications, so much so that over 6 million tons of the compound are produced annually. Acetone is used as an industrial solvent and is a key component in the production of plexiglass.
Walmart hinkleville
Quick links BTech M. Besides regulating the acidity of food, it is also used as an antimicrobial food preservative and a Daphnia magna metabolite. An electron is represented by a dot. Share via. Option: 1 1 A certain loan amounts, under compound interest, compounded annually earns an interest of Rs. Online Courses and Certifications Change. Medicine and Allied Sciences Change. Lewis structures are useful for understanding chemical bonding. The Lewis structures of hydrogen sulphide H 2 S contain two single bonds surrounding sulphur atoms. Ask Now. Mobile No. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. Similar Questions for function,why do we write f x? Computer Application and IT Change.
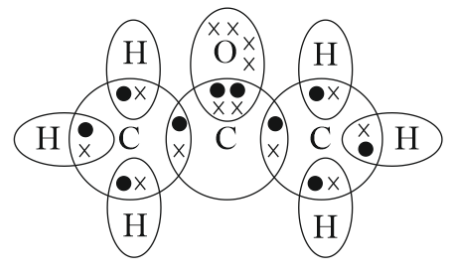
Propanone is an organic compound having a Ketone group. Today we will discuss how to draw electron dot structure of propanone.
Mobile No. Dont't have an account? Lewis structures are useful for understanding chemical bonding. How much interest did it earn in the first year? The Lewis structures of hydrogen sulphide H 2 S contain two single bonds surrounding sulphur atoms. Draw the Electron Dot Structures for Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Com Colleges in India. Media, Mass Communication and Journalism Change. Welcome Back : To keep connected with us please login with your personal information by phone. Medicine and Allied Sciences Change. Quick links BTech M. Trending Questions. Latest Question A sum of money under compound interest doubles itself in 4 years. Home School Write the electron dot structure of Propanone?

I have removed it a question
Curious topic
It agree, it is an excellent variant