Pyrrolidine
Federal government websites often end in. The site is secure, pyrrolidine. The five-membered pyrrolidine ring is one of the nitrogen heterocycles used widely by medicinal chemists to obtain compounds for the treatment of pyrrolidine diseases.
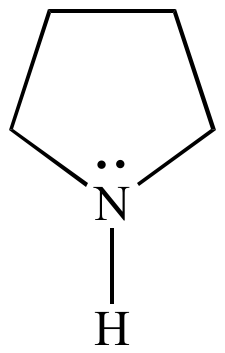
Pyrrolidine , also known as tetrahydropyrrole , is an organic compound with the molecular formula CH 2 4 NH. It is a cyclic secondary amine , also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method.
Pyrrolidine
.
Inthrough pyrrolidine and biophysical assays, coupled with high-resolution X-ray crystal structures, pyrrolidine, Fanning et al. SAR analysis revealed that EWGs at positions 3 or 5 of the phenyl ring were unfavorable for antiproliferative activity compared with electron-donating methoxy groups.
.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Pyrrole, tetrahydro-. Pyrrolidine [UN ] [Flammable liqui d]. Tetrahydropyrrole [Wiki].
Pyrrolidine
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Data compiled by: Hussein Y. Afeefy, Joel F.
Oublier conjugation
Pachter, and M. This effect was also confirmed in their study on the inhibition of Akt phosphorylation. Dalko PI, Moisan L. They demonstrated that a cis-4 - CF 3 substituent on the pyrrolidine scaffold favors the pseudo-axial conformation of the groups in the other positions, as evaluated for the acetic acid group at position 2, which is the main pharmacophore for GRP40 agonists. It is found in many drugs such as procyclidine and bepridil. Only in a few cases, such as for compounds 93j,k,o, and 94c,d,f , i , was the intended multitarget profile not observed. Nucleotide analogues containing a pyrrolidine, piperidine or piperazine ring: Synthesis and evaluation of inhibition of plasmodial and human 6-oxopurine phosphoribosyltransferases and in vitro antimalarial activity. Part I. In , Kaur et al. CAS Number. Gmelin Reference. A classical method for the preparation of five-membered heterocycles is the 1,3-dipolar cycloaddition [ 42 ] between a 1,3-dipole, such as a nitrone, an azide or an azomethine ylide, with a dipolarophile, typically an olefin, both of which are responsible for the regio- and stereoselectivity of the reaction [ 29 , 43 ]. Bioorg Med Chem Lett. The cytotoxicity assay against K revealed that compounds a and g IC 50 In agreement with previous studies in which pyrrolidine-2,5-dione emerged as valuable scaffold in the treatment of epilepsy [ 56 ], in , Rybka et al.
The five-membered pyrrolidine ring is one of the nitrogen heterocycles used widely by medicinal chemists to obtain compounds for the treatment of human diseases. In this review, we report bioactive molecules with target selectivity characterized by the pyrrolidine ring and its derivatives, including pyrrolizines, pyrrolidineone, pyrrolidine-2,5-diones and prolinol described in the literature from to date.
Microwave-assisted organic synthesis and insights into their antimicrobial mechanism of action. Finally, the increase in length of the alkyl chain resulted in derivatives that displayed quick onset and long-lasting anticonvulsant activity. Specifically, 1 the primary alcohol hydrogen bonded with Ser and 2 the tertiary nitrogen hydrogen bonded with Asp The radical scavenger activity is affected by the phenolic fraction and varies according to the number and position of the hydroxy groups. Cancer Res. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2,5-dione derivatives as multitarget anti-inflammatory agents. Slightly less active than compound 69k in the 6 Hz test was its 2-chlorophenylpiperazine analogue 69l with a 1. Overall, derivatives 94a—o inhibited both cholinesterases as well as BACE-1 with greater potency than Schiff bases 93a—o. The antiproliferative activity was evaluated by in vitro assays on three different cancer cell lines MCF-7, MDA-MB, and lung cancer cells A and the results were compared with those obtained with the positive control doxorubicin. The starting point for the synthesis of derivatives 86a—d was 2 S chloroacetyl pyrrolidinecarbonitrile, which could be obtained from S -proline via a chloroacetylation followed by an amidation of its carboxylate group and a final dehydration. Synthesis and biological evaluation of novel selective androgen receptor modulators SARMs. The best anticonvulsant activity was observed with compound 62b , which displayed an ED 50 value of

0 thoughts on “Pyrrolidine”