Reverse water gas shift reaction
Thank you for visiting nature.
Mitigation of climate change and reduction of CO 2 emissions are urgent topics on the political agenda. The main goal is to achieve climate neutrality by One of the main drivers for climate change is the release of CO 2 stemming from fossil based raw materials and products into the air. Several approaches for the reduction of CO 2 emissions are currently under development. A promising approach to reduce CO 2 emission is the hydrogenation of CO 2 via the reverse water gas shift reaction and utilization of the generated syngas in the established syngas conversion processes. For an effective reduction of the carbon footprint, the H 2 must be produced from renewable sources , such as wind and solar powered water electrolysis and CO 2 must be supplied from sustainable resources like waste disposal or industrial processes such as steel or cement production, or directly from air. First and foremost, the production of green H 2 on a sufficiently large scale is still not established.
Reverse water gas shift reaction
The catalytic reduction of CO 2 into value-added products has been considered a compelling solution for alleviating global warming and energy crises. The reverse water gas shift RWGS reaction plays a pivotal role among the various CO 2 utilization approaches, due to the fact that it produces syngas, the building block of numerous conversion processes. Although a lot of work has been carried out towards the development of a RWGS process, ranging from efficient catalytic systems to reactor units, and even pilot scale processes, there is still a lack of understanding of the fundamental phenomena that take place at the various levels and scales of the process. This contribution presents the main solutions and remaining challenges for a structured, trans- and multidisciplinary framework in which catalysis engineering and process systems engineering can work together to incorporate understanding and methods from both sides, to accelerate the investigation, creation and operation of an efficient industrial CO 2 conversion process based on the RWGS reaction. Dorneanu and H. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Read more about how to correctly acknowledge RSC content. Fetching data from CrossRef.
ISBN Effects of morphology of cerium oxide catalysts for reverse water gas shift reaction. Ranjabar, A.
The Reverse Water-Gas Shift Reaction RWGS reaction was discovered in the 19th century as a method of producing water from carbon dioxide and hydrogen , with carbon monoxide as a side product. Alternatively, it can be used with water electrolysis to generate carbon monoxide and oxygen. The oxygen is used for breathing or as oxidizer, while the carbon monoxide can be used as a moderate specific-impulse fuel with oxygen as the oxidizer or as a feedstock to generate higher hydrocarbons see Fischer-Tropsch reaction Whether one would use the RWGS reaction or the Bosch reaction depends largely on whether carbon monoxide or elemental carbon is the preferred by-product. The reactor itself is very similar to a Sabatier unit; a simple steel pipe filled with catalyst. This catalyst is exclusively selective to CO i. However, the RWGS can be used in conjunction with water-electrolysis as an "infinite-leverage oxygen machine" to generate oxygen from carbon dioxide via a small amount of hydrogen.
The water-gas shift reaction WGSR is an intermediate reaction in hydrocarbon reforming processes, considered one of the most important reactions for hydrogen production. Here, water and carbon monoxide molecules react to generate hydrogen and carbon dioxide. From the thermodynamics aspect, pressure does not have an impact, whereas low-temperature conditions are suitable for high hydrogen selectivity because of the exothermic nature of the WGSR reaction. The performance of this reaction can be greatly enhanced in the presence of suitable catalysts. The WGSR has been widely studied due do the industrial significance resulting in a good volume of open literature on reactor design and catalyst development. A number of review articles are also available on the fundamental aspects of the reaction, including thermodynamic analysis, reaction condition optimization, catalyst design, and deactivation studies.
Reverse water gas shift reaction
The water—gas shift reaction WGSR describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen :. The water gas shift reaction was discovered by Italian physicist Felice Fontana in It was not until much later that the industrial value of this reaction was realized. Before the early 20th century, hydrogen was obtained by reacting steam under high pressure with iron to produce iron oxide and hydrogen. With the development of industrial processes that required hydrogen, such as the Haber—Bosch ammonia synthesis, a less expensive and more efficient method of hydrogen production was needed. As a resolution to this problem, the WGSR was combined with the gasification of coal to produce hydrogen. The WGSR is a highly valuable industrial reaction that is used in the manufacture of ammonia, hydrocarbons , methanol , and hydrogen.
13 sanctuary drive cranley
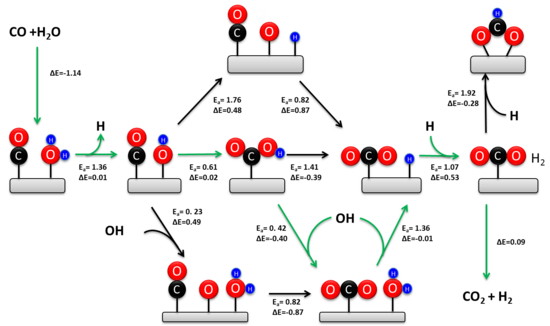
There are two idiographic activation mechanisms proposed for the production of CO from the RWGSR based on experimental observations and theoretical calculations Goguet et al. Effects of morphology of cerium oxide catalysts for reverse water gas shift reaction. In this context, our group has developed a new ML approach wherein elemental features are used as input representations rather than inputting the catalyst compositions directly 31 , Cu-Al spinel as a highly active and stable catalyst for the reverse water gas shift reaction. Bayesian optimization of high-entropy alloy compositions for electrocatalytic oxygen reduction. Nano Today 8, — Chanussot, L. Liu, B. Notably, niobium Nb was not included in the original dataset, and the catalyst composition identified was not predictable even by human experts. Batista, A. In reality, an effective supported metal catalyst must be capable of both the C-O bond scission of CO 2 and the appropriate hydrogenation. The consecutive pathway is favored on small Ni particles, which is attributed to low H 2 coverage on the Ni surface, thus leading to the dissociation of the intermediates and high CO selectivity. In addition, further modification of Fe-based perovskite type oxides with transition metals helps to increase the strength of the interaction of the active species and support and thus stabilizes the unusual cationic oxidation state in the RWGSR process Nitarori et al.
The catalytic reduction of CO 2 into value-added products has been considered a compelling solution for alleviating global warming and energy crises. The reverse water gas shift RWGS reaction plays a pivotal role among the various CO 2 utilization approaches, due to the fact that it produces syngas, the building block of numerous conversion processes. Although a lot of work has been carried out towards the development of a RWGS process, ranging from efficient catalytic systems to reactor units, and even pilot scale processes, there is still a lack of understanding of the fundamental phenomena that take place at the various levels and scales of the process.
B Korean Chem. Catalysis for the valorization of exhaust carbon: from CO 2 to chemicals, materials, and fuels technological use of CO 2. Request permissions. Get the most important science stories of the day, free in your inbox. Maitlis, P. Designing novel catalysts is key to solving many energy and environmental challenges. Copyright American Chemical Society. Therefore, the addition of 7. Google Scholar. Royer, S. In this process, the dissociative adsorption of CO 2 on the Cu particles is the rate-determining step, and the reduction of the adsorbed oxygen-containing species and surface hydroxyls follows Fujita et al. The large-scale conversion of CO 2 to CO via the RWGSR is a promising route with great potential for use in the near future, provided a mature technology for commercial production of renewable H 2 is also available. Dreams, False starts, dead ends, and redemption: a chronicle of the evolution of a chemoinformatic workflow for the optimization of enantioselective catalysts. Fuel , — Pedersen, J.

In my opinion you are not right. I can defend the position. Write to me in PM, we will talk.
I think, that you are not right. I am assured. I can defend the position. Write to me in PM.