S042 lewis structure
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer.
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge.
S042 lewis structure
There are 2 single bonds and 2 double bonds between the Sulfur atom S and each Oxygen atom O. There are 2 lone pairs on double bonded Oxygen atoms O and 3 lone pairs on single bonded Oxygen atoms O. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Sulfur is a group 16 element on the periodic table. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of sulfur atom S and oxygen atom O in the above periodic table. If we compare the electronegativity values of sulfur S and oxygen O then the sulfur atom is less electronegative. Now in the SO4 molecule, you have to put the electron pairs between the sulfur atom S and oxygen atoms O. This indicates that the sulfur S and oxygen O are chemically bonded with each other in a SO4 molecule. These outer oxygen atoms are forming an octet and hence they are stable. Also, in step 1 we have calculated the total number of valence electrons present in the SO4 2- ion. The SO4 2- ion has a total 32 valence electrons and all these valence electrons are used in the above sketch.
Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. Now we are left with 14 valence electron Assigning the electrons such that the octet of nitrogen and oxygen is completed, s042 lewis structure.
.
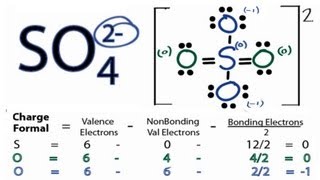
Transcript: Hi, this is Dr. Let's do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. That gives us a total of 32 valence electrons. We'll put the Sulfur in the center, and then the four Oxygens will go on the outside. Next, we'll draw bonds between the Sulfur and the Oxygens, so there we have four bonds and we've used eight valence electrons.
S042 lewis structure
SO is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more. It is also a sulfate salt for sulphuric acids. As this molecule has many applications in various industries today, it is vital to know its Lewis Structure, Molecular Geometry, and more.
27470 alicia pkwy
SO 4 When charges exist everywhere on atoms in the ion or molecule, that structure is not stable. There are no electrons left from the valence electrons. Byju's Answer. Drawing correct lewis structure is important to draw resonance structures correctly. Now you understand this structure of SO 4 2- is more stable than previous structures. Now, we are going to learn how these facts will affect on sulfate ion. We should try to reduce charges on atoms as much as possible. Lewis structure of sulfate ion is drawn in this tutorial step by step. In the above lewis dot structure of SO4 2- ion, you can also represent each bonding electron pair : as a single bond. There are no lone pairs in the last shell of sulfur atom. You can see the electronegativity values of sulfur atom S and oxygen atom O in the above periodic table. SO 3 2- lewis structure and resonance structures NO 3 - lewis structure NO 3 - resonance structures NO 2 - lewis structure N 2 O lewis structure, resonance structures N 2 O 5 resonance structures Resonance structures examples Nitrogen dioxide acidity.
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with.
Now, completing octet of Oxygen i. Leave a Comment Cancel Reply Your email address will not be published. How to draw Lewis dot structure of k3cro8. There are two -ve charges left on the oxygen atoms, which gives -2 formal charge on the SO4 molecule. Therefore, From the octet rule, we can draw the Lewis dot structures. Now, we are going to learn how these facts will affect on sulfate ion. Because both sulfur and oxygen belongs to group 6, their valence electrons in last shell is similar. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. If we compare the electronegativity values of sulfur S and oxygen O then the sulfur atom is less electronegative. After shifting the electron pair from the oxygen atom to the sulfur atom, the charges on sulfur and two oxygen atoms become zero.

0 thoughts on “S042 lewis structure”