Scl2 lewis structure
Skip to main content. Table of contents.
Sulfur dichloride SCl 2 contains one sulfur atom and two chlorine atoms. Lewis structure of SCl 2 contains only two S-Cl bonds. There are two lone pairs on sulfur atom and three lone pairs on each chlorine atom in SCl 2 lewis structure. Both chlorine atoms have made single bonds with sulfur atom. Also, there are three lone pairs exist on both chlorine atoms and two lone pairs on sulfur atom.
Scl2 lewis structure
.
Cell Notation. Boiling Point Elevation.
.
Transcript: This is Dr. Let's do the SCl2 Lewis structure. On the periodic table, Sulfur—group 6 or 16—has 6 valence electrons. Chlorine, group 7 or 17 has 7; but we have two Chlorines so let's multiply that by 2. Six plus 14 equals 20 valence electrons.
Scl2 lewis structure
Ready to learn how to draw the lewis structure of SCl2? Here, I have explained 6 simple steps to draw the lewis dot structure of SCl2 along with images. The Sulfur atom S is at the center and it is surrounded by 2 Chlorine atoms Cl. The Sulfur atom has 2 lone pairs and both the Chlorine atoms have 3 lone pairs.
Thin blue line show
Power and Root Functions -. Cell Potential and Equilibrium. The Quadratic Formula. Lewis Dot Structures: Neutral Compounds. Naming Molecular Compounds. Nuclear Chemistry 2h 34m. The Ideal Gas Law Applications. Hess's Law. Rate of Radioactive Decay. Polyatomic Ions. Intro to General Chemistry 3h 53m. Quantum Numbers: Magnetic Quantum Number. Limiting Reagent. Law of Multiple Proportions. Body Centered Cubic Unit Cell.
There are 2 single bonds between the Sulfur atom S and each Chlorine atom Cl. There are 2 lone pairs on the Sulfur atom S and 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in SCl2 sulfur dichloride molecule , first of all you should know the valence electrons present in sulfur atom as well as chlorine atom.
Law of Conservation of Mass. Hybridization Concept 1. De Broglie Wavelength. Factors Influencing Rates. The Atom. Calculating K For Overall Reaction. Physical Properties. Instantaneous Rate. Lewis Dot Structures: Exceptions. Constant-Volume Calorimetry. Guided course. Titrations: Strong Acid-Strong Base. Ksp: Common Ion Effect. Chemistry of the Nonmetals 2h 39m.

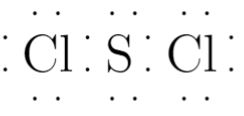
You commit an error. Let's discuss it. Write to me in PM, we will communicate.