Sf4 molecular geometry
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons. Fluorine is located in Group 17 and has seven valence electrons, sf4 molecular geometry.
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown.
Sf4 molecular geometry
.
Fluorine is located in Group 17 and has seven valence electrons. Sulphur is a periodic table group VIA element with six electrons in its final shell valence shell. The five valence atomic orbitals of the S atom are hybridised in the middle to produce five sp3d hybrid orbitals, sf4 molecular geometry.
.
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest neighbor, neon. Sulfur and fluorine will combine to form four S-F single bonds. Sulfur will use four valence electrons to bond with the four fluorine atoms. Hence, it will have one lone pair of electrons, while each fluorine atom will have six []. Lewis structure is used to show the bond formation in sulfur tetrafluoride. Sulfur is the least electronegative of the two. So, it will lie at the center of the molecule.
Sf4 molecular geometry
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons. Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons. During the formation of SF4, the sulphur atom will form bonds with each of fluorine atoms where 8 of valence electrons are used. Meanwhile, the four fluorine atoms will have 3 lone pairs of electrons in its octet which will further utilize 24 valence electrons. In addition, two electrons will be kept as lone pair in the sulphur atom.
Best semi auto shotgun 2021
Let us learn about the SF4 molecular geometry and bond angles. Types of Impurity Defects. Get subscription. Reserved Seats. Access more than. Allotment of Examination Centre. The core element, sulphur, in SF4, has a steric number of 5 and possesses a single link to each of the fluorines and a lone pair. Sulphur Tetrafluoride contains 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the core atom in its Lewis structure. Ans : S — atom in SF 4 contain a single electron pair. Ans : Draw the SF The lone pair will most likely be in one of the three equatorial positions, which is the most open zone imaginable, rather than one of the two axial places. Challenge Yourself Everyday.
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles.
Hepatic Portal System. About Contact. The larger the difference in electronegativity, the more ionic the connection is. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. Sulphur Tetrafluoride contains 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the core atom in its Lewis structure. The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest neighbor, neon. Let us learn about the SF4 molecular geometry and bond angles. Ans : S — atom in SF JEE Application Process. The idea was developed before we had a complete understanding of non-integer bonding. The five valence atomic orbitals of the S atom are hybridised in the middle to produce five sp3d hybrid orbitals. Enthalpy of Neutralisation.

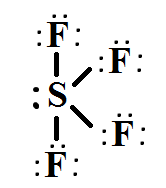
Almost the same.
In it something is also to me this idea is pleasant, I completely with you agree.