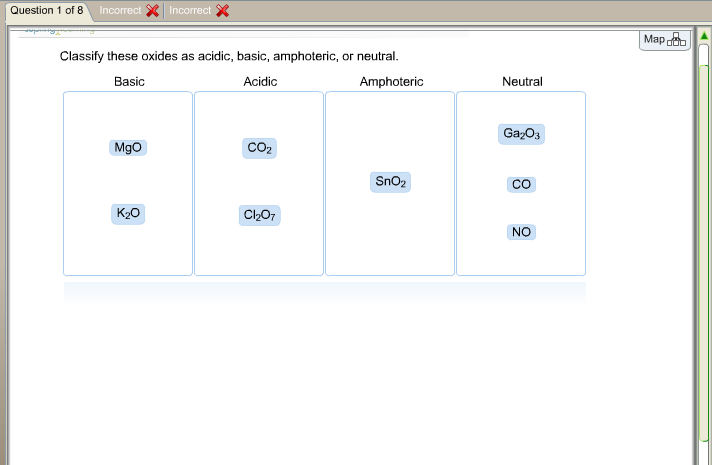
Sno2 is acidic or basic
The inorganic compound tin IV oxide, also known as stannic oxide, has the formula SnO2. Cassiterite is a tin oxide mineral, SnO2, and it is the most common tin ore.
In this quick video, Mike Jones explains the properties of the period three oxides. You'll see examples of acidic, basic, and amphoteric oxides. You'll also learn how their bonding affects their acid-base character. This is a great quick video for exploring oxides! In this video, the Chemistry Demo Lab at Ohio State shows you how different oxides can be acidic or basic.
Sno2 is acidic or basic
.
Your Mobile number and Email id will not be published.
.
This page briefly examines the oxides of carbon, silicon, germanium, tin and lead. It concentrates on the structural differences between carbon dioxide and silicon dioxide, and on the trends in acid-base behavior of the oxides down Group 4. The physical properties of carbon dioxide differ significantly from those of silicon dioxide also known as silicon IV oxide or silica. Carbon dioxide is a gas whereas silicon dioxide is a hard, high-melting solid. The other dioxides in Group 4 are also solids, making the structure of carbon dioxide the anomaly. The fact that carbon dioxide is a gas indicates that it consists of small, simple molecules. Carbon can form these molecules because it can form double bonds with oxygen. None of the other elements in Group 4 form double bonds with oxygen, so their oxides adopt completely different structures.
Sno2 is acidic or basic
This page discusses the reactions of the oxides of Period 3 elements sodium to chlorine with water, and with acids or bases where relevant as before, argon is omitted because it does not form an oxide. Acidity increases from left to right, ranging from strongly basic oxides on the left to strongly acidic ones on the right, with an amphoteric oxide aluminum oxide in the middle. An amphoteric oxide is one which shows both acidic and basic properties. This trend applies only to the highest oxides of the individual elements see the top row of the table , in the highest oxidation states for those elements. The pattern is less clear for other oxides. Non-metal oxide acidity is defined in terms of the acidic solutions formed in reactions with water—for example, sulfur trioxide reacts with water to forms sulfuric acid.
Medley crossword clue
For example, consider carbon dioxide , CO2. Difference Between Accuracy And Precision. You'll see examples of acidic, basic, and amphoteric oxides. Specifically, the alkali and alkaline-earth metal oxides are very basic. Way back, we studied oxoanions. Carbonic Acid H 2 CO 3. Urethane C 3 H 7 NO 2. Neutral Oxides A handful of oxides are neutral. And our reasoning is correct! We produce carbonic acid!
Tin IV oxide , also known as stannic oxide , is the inorganic compound with the formula SnO 2. The mineral form of SnO 2 is called cassiterite , and this is the main ore of tin. It is a colourless, diamagnetic , amphoteric solid.
Go to Topic. Many oxides form acids. Let's look at tin IV oxide as an example. Chemistry Metals and Nonmetals Tin Oxide. Dipole Moment. Is SnO2 acidic or basic? An oxide is a molecule or compound with an oxygen atom bonded to one element. FREE Signup. Cassiterite is a tin oxide mineral, SnO2, and it is the most common tin ore. Who Invented The Periodic Table. They do not react with either acids or bases. Specifically, the alkali and alkaline-earth metal oxides are very basic.

Thanks for the help in this question.
This topic is simply matchless