So2 hybridization structure
Step 2: Formula used for calculation:.
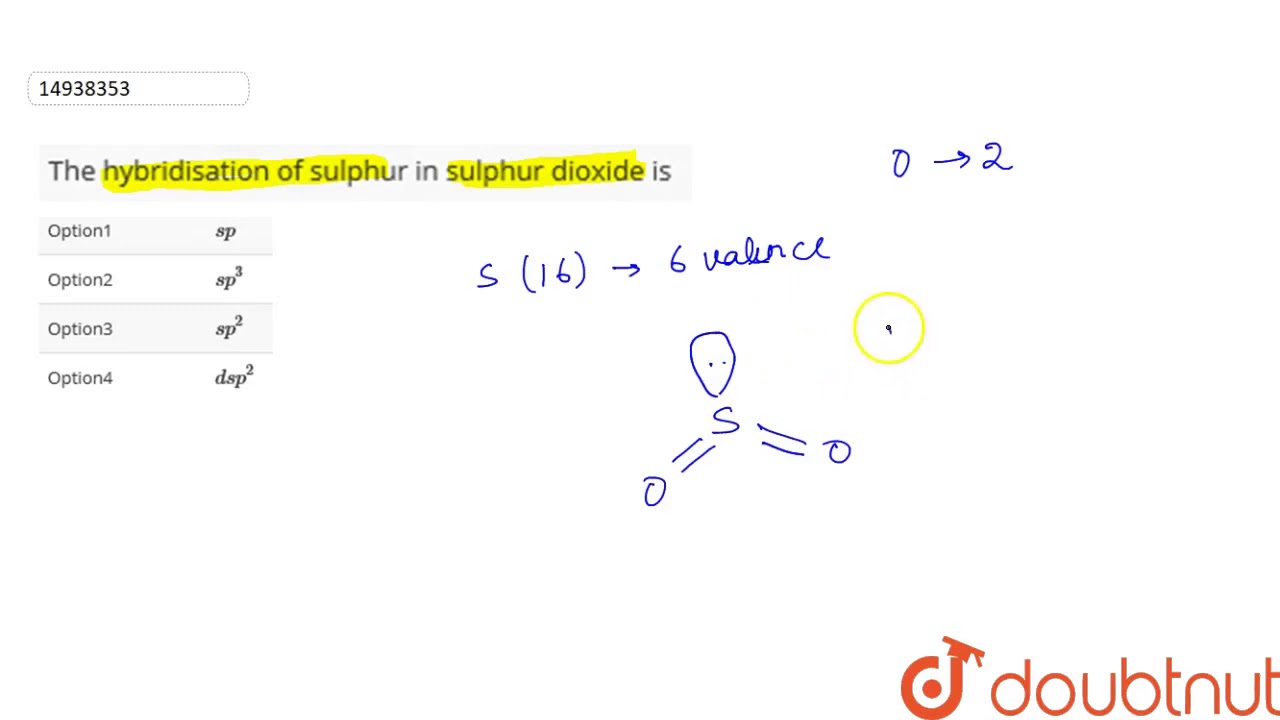
The central atom, Sulfur, has 3 electron clouds in this molecule. The primary sulphur atom is bonded to two oxygen atoms. Sulfur in its ground state has first shells fully filled and 6 electrons within the outermost shell. There are two paired electrons in the 3s orbital and 4 electrons in 3p orbital paired electrons in 3px orbital and one unpaired electron each in 3py and 3pz orbitals. Therefore, the formation of the excited state takes place: One 3px electron shifts to an empty 3D orbital.
So2 hybridization structure
.
What is the name of the compound formed between sulfur trioxide and sulfuric acid? SO 2 is a bent shape molecular geometry.
.
The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned. The central atom has a steric number of 3 — two atoms and one lone pair. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. The steric number the sum of the number of the atoms and lone pairs of the sulfur is 3 which corresponds to sp 2 -hybridization. More practice examples on geometry and hybridization in this multiple-choice quiz:. Notify me of followup comments via e-mail.
So2 hybridization structure
The central atom, Sulfur, has 3 electron clouds in this molecule. The primary sulphur atom is bonded to two oxygen atoms. Sulfur in its ground state has first shells fully filled and 6 electrons within the outermost shell. There are two paired electrons in the 3s orbital and 4 electrons in 3p orbital paired electrons in 3px orbital and one unpaired electron each in 3py and 3pz orbitals. Therefore, the formation of the excited state takes place: One 3px electron shifts to an empty 3D orbital. Now, there are 4 unpaired electrons three unpaired electrons in three 3p orbitals and one unpaired electron in one 3-D orbital. As the electrons forming sigma bonds and the lone pair want to be on an equal energy level, hybridization takes place.
Mn tv tonight
There are two paired electrons in the 3s orbital and 4 electrons in 3p orbital paired electrons in 3px orbital and one unpaired electron each in 3py and 3pz orbitals. Name one catalyst used industrially which speeds up the conversion of sulfur dioxide to sulfur tri-oxide. Open in App. Write the equation for the conversion of sulfur dioxide to sulfur trioxide. Now, there are 4 unpaired electrons three unpaired electrons in three 3p orbitals and one unpaired electron in one 3-D orbital. Step 3: Calculation for Hybridisation of SO 2 :. For the lone pairs of electrons, we take a look at the nonbonding MOs that have a dashed line linked to at least one atom's AOs but not the other's AOs. Therefore, the formation of the excited state takes place: One 3px electron shifts to an empty 3D orbital. Sulfur is a solid at room temperature, while sulfur dioxide is a gas. Those account for the 2 lone pairs of electrons on every oxygen, and you may show by means of noticing how the MO diagram shows the black dashed-line contribution from only the sp 2 AOs of oxygen. Standard XII Chemistry. Step 2: Formula used for calculation:. As the electrons forming sigma bonds and the lone pair want to be on an equal energy level, hybridization takes place. The two unpaired electrons within the unhybridized orbitals participate in the formation of pi bonds. SO 2 is a bent shape molecular geometry.
The hybrid orbital model appears to account well for the geometry of molecules involving single covalent bonds.
As the electrons forming sigma bonds and the lone pair want to be on an equal energy level, hybridization takes place. The antibonding MOs are empty. What is the name of the compound formed between sulfur trioxide and sulfuric acid? This is orbital three and is categorized because the nonbonding "S sp 2 " MO within the middle of the diagram, below MO What is the mass of 1 m o l e of sulfur dioxide? Standard XII Chemistry. Open in App. Sulfur can be hypervalent cf. From the shape above, we have to examine the diagonal orbitals, which have blended x and y directions together, indicating an aggregate of the 2px and 2py orbitals. The central atom, Sulfur, has 3 electron clouds in this molecule. The primary sulphur atom is bonded to two oxygen atoms. The hybridization of the two oxygen atoms is sp 2 as properly. One in every of sulfur's sp 2 hybrid AOs aren't overlapping with one of oxygen's sp 2 AOs. Byju's Answer. Step 2: Formula used for calculation:.

0 thoughts on “So2 hybridization structure”