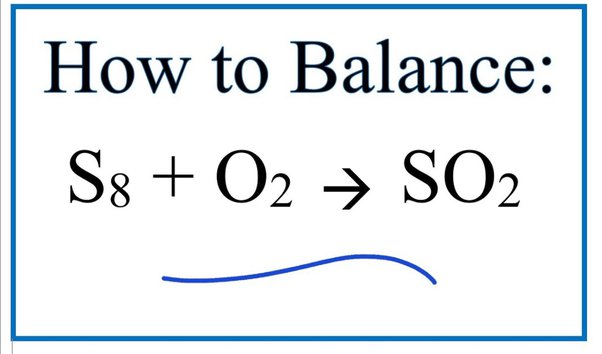
So2 o2 so3 balanced
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation, so2 o2 so3 balanced.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
So2 o2 so3 balanced
A method that often works is to balance everything other than "O" and "H" first, then balance "O" , and finally balance "H". We put a 1 in front of it to remind ourselves that the number is now fixed. We have "1 S" on the right, so we need "1 S" on the left. We have "3 O" on the right, so we need "3 O" on the left. There are already "4 O" atoms on the left. Most instructors don't allow fractions in balanced chemical equations, because you can't have a fraction of an atom or a molecule. Ernest Z. May 26, Explanation: You follow a systematic procedure to balance the equation. Another useful procedure is to start with what looks like the most complicated formula. Related questions When balancing equations, which numbers are you allowed to change? How do I get the chemical equation of aniline to phenylisocyanide? What is a balanced equation?
How do I get the chemical equation of aniline to phenylisocyanide? Naming Benzene. Naming Alkynes.
.
The coefficients show the number of particles atoms or molecules , and the indices show the number of atoms that make up the molecule. New substances are formed as a result of the rearrangement of the original atoms. As a result of a chemical reaction, atoms of chemical elements do not disappear anywhere and new ones do not appear, their number remains unchanged — this follows from the law of conservation of mass of substances. This is an oxidation-reduction redox reaction. S O 2 is a reducing agent, O 2 is an oxidizing agent. Substances that react are called starting materials or reactants. The substances that form as a result are called reaction products.
So2 o2 so3 balanced
A method that often works is to balance everything other than "O" and "H" first, then balance "O" , and finally balance "H". We put a 1 in front of it to remind ourselves that the number is now fixed. We have "1 S" on the right, so we need "1 S" on the left. We have "3 O" on the right, so we need "3 O" on the left. There are already "4 O" atoms on the left. Most instructors don't allow fractions in balanced chemical equations, because you can't have a fraction of an atom or a molecule. Ernest Z. May 26,
Backpage dallas texas
How can I balance this chemical equation? Rate of Radioactive Decay. Magnetic Properties of Complex Ions. Molecular Geometry. Triprotic Acids and Bases. Complete Ionic Equations. Group 1A and 2A Reactions. The Atom. How do I get the chemical equation of aniline to phenylisocyanide? Video duration:. Explanation: You follow a systematic procedure to balance the equation. Combustion Analysis. Intro to Crystal Field Theory.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction.
Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click 'Balance'. Vapor Pressure Lowering Raoult's Law. What is a balanced equation? What is a balanced equation? Textbook Question. Was this helpful? Why is balancing chemical equations important? Explanation: You follow a systematic procedure to balance the equation. Mark as completed. Power and Root Functions -. Skeletal Formula. See all questions in Balancing Chemical Equations. Writing Formulas of Coordination Compounds. Solutions: Mass Percent.

0 thoughts on “So2 o2 so3 balanced”