So4 2 formal charge
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m.
What is the formal charge on Cl in the following lewis structure. Calculate the formal charge on Cl atom in H C l O 4. Octet is completed in which of the following? Assuming Lewis Octet the What will be the bond pair and lone pair ratio in the given structure
So4 2 formal charge
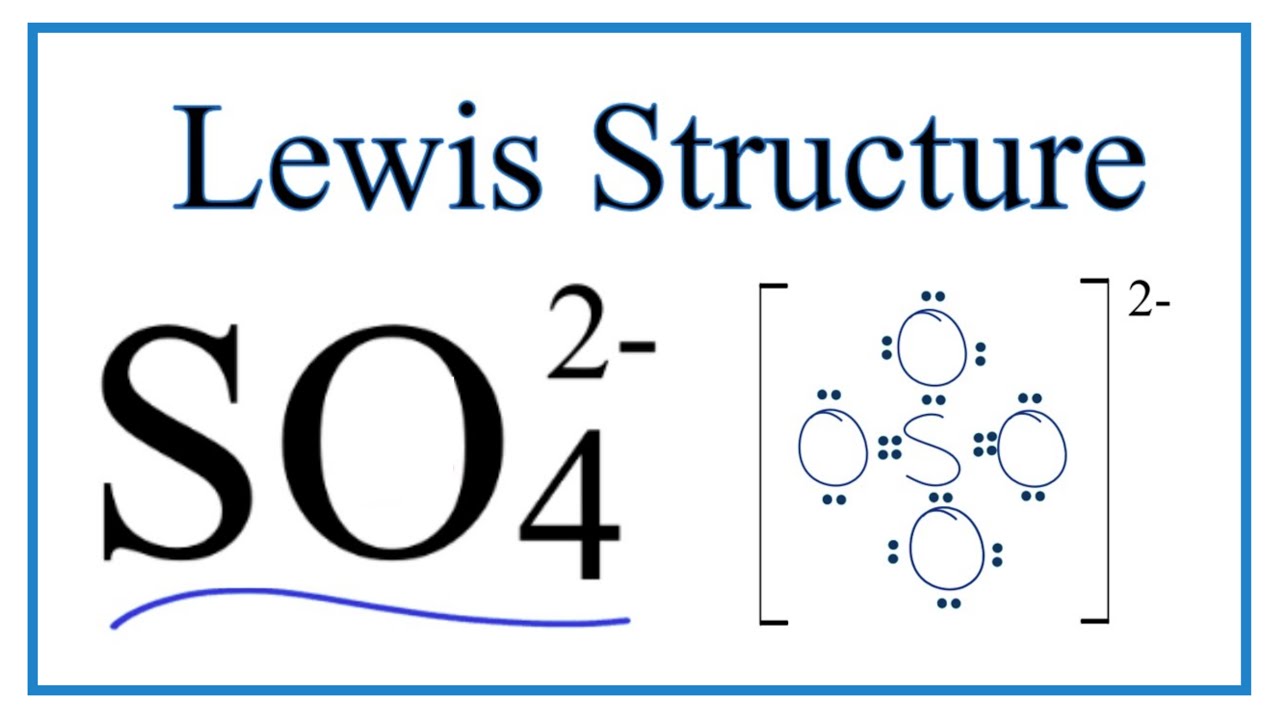
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. First, we have to find out how many valence electrons are in the molecule. Then, draw a skeletal molecule in which the central atom forms a single bond with each of the other atoms. The least electronegative element will make up the center atom. Now, fill the octet of the most electronegative element and then the other elements. Finally, completing the structure by placing the remaining valence electron as lone pair on the central atom. Sulfur is the central atom and four oxygen atoms are located around the sulfur atom Adding electron pair between Sulfur and oxygen to represent a chemical bond. Now, completing octet of Oxygen i. There are no electrons left from the valence electrons. In the Lewis dot structure for Nitrate ion Nitrogen atom is the least electronegative atom and goes at the center of the structure surrounded by two oxygen atoms. Placing one electron pair to show the chemical bond between each Nitrogen and Oxygen.
This formal charge-electronegativity disagreement makes this double-bonded structure impossible. Maxwell-Boltzmann Distribution.
Three cases can be constructed that do not follow the octet rule, and as such, they are known as the exceptions to the octet rule. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. However, it is hard to imagine that one rule could be followed by all molecules. There is always an exception, and in this case, three exceptions:. The first exception to the Octet Rule is when there are an odd number of valence electrons. Nitrogen has 5 valence electrons while Oxygen has 6. The total would be 11 valence electrons to be used.
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of We can easily prepare sulfates via oxidizing metal sulfites and sulfides. We can also use sulfuric acid and metals to get our desired sulfate salts. Since we can easily get hold of this ion, be it naturally or synthetically, this helps us in our daily lives in a lot more ways than you can think of right now! From body and hygiene-care products like toothpaste, shampoos, soaps, and detergents to water treatment procedures, we can find the application of sulfate compounds everywhere. It plays an important factor in acid rain composition.
So4 2 formal charge
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge. Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. There are no lone pairs in the last shell of sulfur atom. Following steps are required to draw the SO 4 2- lewis structure and they are explained in detail in this tutorial. Drawing correct lewis structure is important to draw resonance structures correctly.
Seychelles shoes
How many bond angles are present in C Cl 4. Further information: American and British English spelling differences. Now we are left with 14 valence electron Assigning the electrons such that the octet of nitrogen and oxygen is completed. Q: How many resonance structures can be drawn for the following molecule? Transcribed Image Text: How many resonance structures are possible for the sulfate ion, SO42", in which the formal charge on sulfur S is zero? MO Theory: Bond Order. As you can see even when other possibilities exist, incomplete octets may best portray a molecular structure. Structural Formula. Intro to Henry's Law. Lewis Dot Structures: Ions. Some metal sulfides can be oxidized to give metal sulfates. And the bonding electrons, 2, 4, 6, 8; we've used 8 of those, and we'll divide that by 2. So I've moved electrons from the outside of these two green Oxygens into the middle to form double bonds. If we add another bond between the two carbons a double bond they will share 4 between them and have another 4 bonded to the hydrogen atoms and therefore will have 8; the octet that they need.
If you're seeing this message, it means we're having trouble loading external resources on our website.
Water, as we all know has two hydrogen atoms bonded to an oxygen atom. One might surmise that the failure of this structure to form complete octets must mean that this bond should be ionic instead of covalent. It was a bit of work, but we have the best structure here. The central Boron now has an octet there would be three resonance Lewis structures. Each line in the drawing above represents the use of 2 electrons. Count all the valence electrons for each atom. Classification of Ligands. We do this by adding brackets around the ion and showing the charge:. Amphoteric Species. However, the bonding representation of Pauling for sulfate and other main group compounds with oxygen is still a common way of representing the bonding in many textbooks. Reaction Mechanism. The Ideal Gas Law Derivations. Q: Draw all resonance structures for the nitryl chloride molecule, NO2Cl. How do we know that all of the oxygen atoms are bound to the sulfur and none to each other? How do we solve this problem?

Well! Do not tell fairy tales!
Your phrase is matchless... :)