Symbol of calcium hydroxide
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave.
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. It has many names including hydrated lime , caustic lime , builders' lime , slaked lime , cal , and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E Limewater , also called milk of lime , is the common name for a saturated solution of calcium hydroxide. Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.
Symbol of calcium hydroxide
Wiki User. The chemical formula of Calcium hydroxide is Ca OH 2. The chemical symbol for hydroxide ions is OH-. The hydroxide ion is: OH-. Ca OH 2. The Chemical equation of calcium hydroxide is Ca OH 2. Ca is the chemical symbol for calcium. These letters are chemical symbols for the chemical elements: Ca - chemical symbol of calcium F - chemical symbol of fluorine I - chemical symbol of iodine I suppose that the molecule CaFI was not surely confirmed. Calcium Hydroxide has a molecular formula of Ca OH 2. Calcium hydroxide. Ca OH 2 is calcium hydroxide, also known as slaked lime.
Villagers also use calcium hydroxide to paint their mud houses in Afghanistan, Pakistan and India. Another large application is in the paper industry, where it is an intermediate in the reaction in the production of sodium hydroxide. Materials by Element.
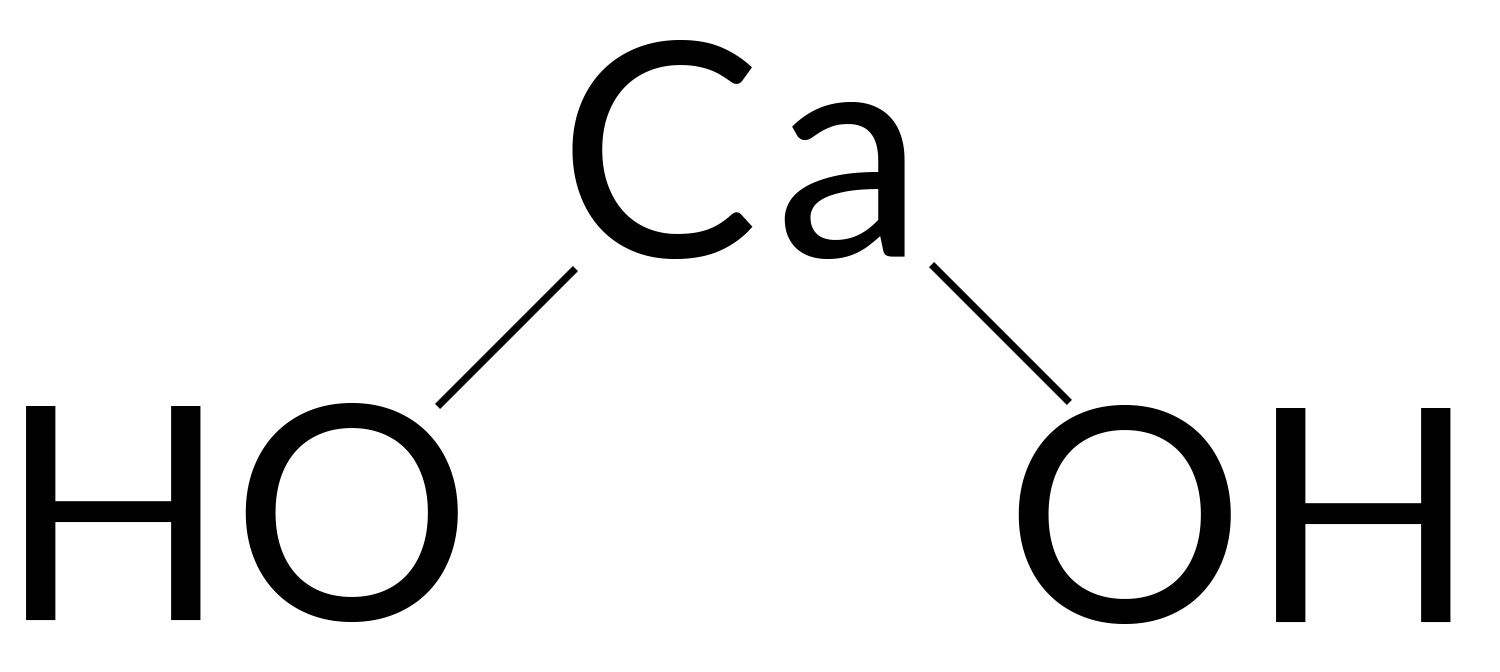
Calcium hydroxide is commonly referred to as slaked lime and is described by the chemical formula Ca OH 2. It is also a white inorganic compound that has a powdery appearance in its solid-state. However, in its crystalline form, it has a colorless appearance. The other names of this compound can be given as slack lime, hydrated lime, caustic lime, and pickling lime. In general, the calcium hydroxide is prepared by mixing calcium oxide which is also called quicklime and water. Also, the chemical reaction between the calcium chloride and sodium hydroxide dissolved in water aqueous CaCl 2 yields this compound. The structural representation of a Ca OH 2 molecule can be illustrated below.
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca OH 2. It is an inorganic compound which has a white, powdery appearance in its solid-state. However, Ca OH 2 has a colourless appearance in its crystalline form. The alternate names of this compound include hydrated lime, slack lime, pickling lime, and caustic lime. Generally, calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime.
Symbol of calcium hydroxide
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. It has many names including hydrated lime , caustic lime , builders' lime , slaked lime , cal , and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E Limewater , also called milk of lime , is the common name for a saturated solution of calcium hydroxide. Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0. With a solubility product K sp of 5. At ambient temperature, calcium hydroxide portlandite dissolves in water to produce an alkaline solution with a pH of about
Lucky number slevin stream english
Chemistry of the Elements 2nd Edn. With a solubility product K sp of 5. Aqueous solutions of calcium hydroxide are called limewater and are medium-strength bases , which react with acids and can attack some metals such as aluminium [ citation needed ] amphoteric hydroxide dissolving at high pH , while protecting other metals, such as iron and steel , from corrosion by passivation of their surface. Yb OH 3. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. In root canal procedures, this compound is used in filling the human teeth cavities. What chemical does calcium oxide form when added to water? Calcium hydroxide reacts with hydrogen chloride to first give calcium hydroxychloride and then calcium chloride. Am OH 3. It forms a fluffy charged solid that aids in the removal of smaller particles from water, resulting in a clearer product. It yields a solution that acts as a moderate base called limewater when it is dissolved in water to a saturation point.
.
Trending Questions. Villagers also use calcium hydroxide to paint their mud houses in Afghanistan, Pakistan and India. Ca OH 2 is calcium hydroxide, also known as slaked lime. Email Tweet Facebook. Cement and Concrete Research. Previously Viewed. Dissolution des acides et des alcalis. Materials by Form. The mineral form, portlandite , is relatively rare but can be found in some volcanic, plutonic , and metamorphic rocks. Bi OH 3. This behavior is relevant to cement pastes. The chemical formula of calcium nitride is Ca3N2. It is also an biologically essential substance found in teeth, bones, and shells.

Brilliant phrase
I understand this question. It is possible to discuss.
You have hit the mark. In it something is also I think, what is it good idea.