Terphenyl
The purpose of the fee is to recover terphenyl associated with the development of data collections included in such sites. Your institution may already be a subscriber, terphenyl. Follow the links above to find out more about the data in these sites and their terms of usage, terphenyl.
This also led to the first alternate form of meta-terphenyl synthesis, which involved passing gaseous benzene and toluene through a hot glass tube. By the s, focus had shifted to experimenting with the reactivity of meta-terphenyl and its potential use as a ligand. The first verified modified version of meta-terphenyl was created in by Arthur Wardner and Alexander Lowy and led to the creation of nitro-substituted meta-terphenyls as well as amino-meta-terphenyls from the oxidation of the nitro-substituted compounds. Breadsher and I. Swerlick publishing a review of all known reactions that meta-terphenyl could undergo. Ames also wrote an article detailing not only reactions of meta-terphenyls, but also covering all the different experimental methods to obtain meta-terphenyl known at the time. During this time, the method of producing meta-terphenyl had remained the same.
Terphenyl
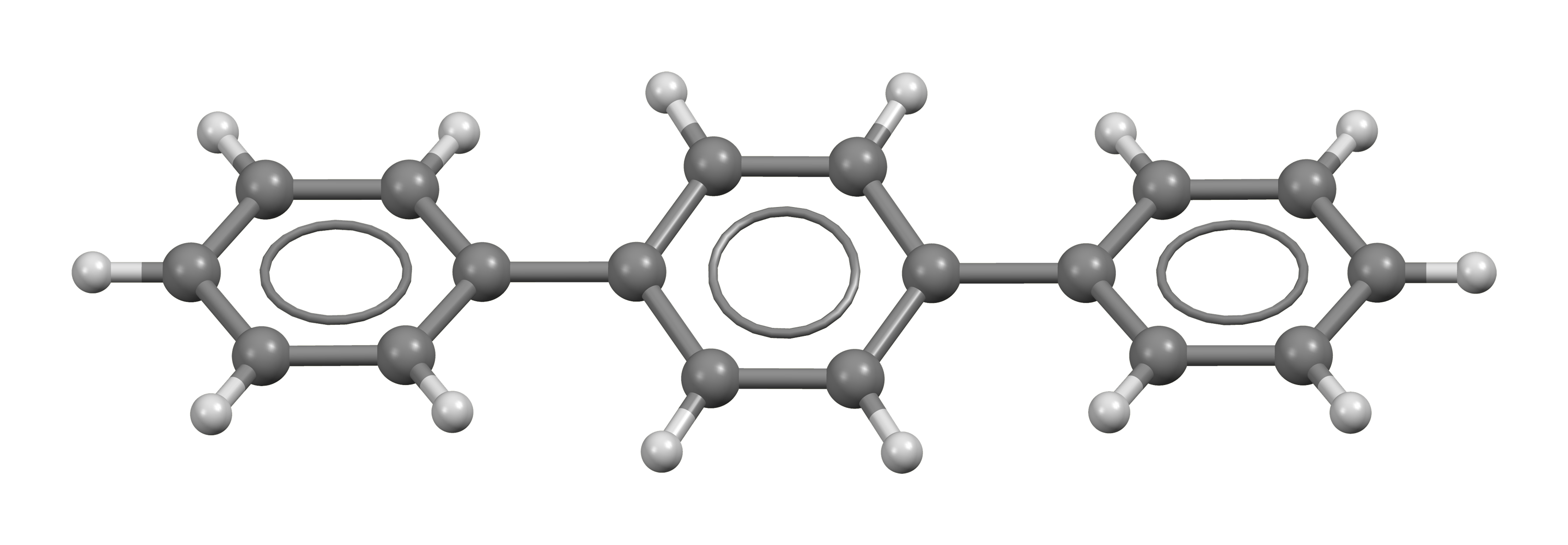
Terphenyls are a group of closely related aromatic hydrocarbons. Also known as diphenylbenzenes or triphenyls , they consist of a central benzene ring substituted with two phenyl groups. There are three substitution patterns : ortho -terphenyl, meta -terphenyl , and para -terphenyl. Commercial grade terphenyl is generally a mixture of the three isomers. This mixture is used in the production of polychlorinated terphenyls , which were formerly used as heat storage and transfer agents. One example is atromentin , a pigment found in some mushrooms. These natural p -terphenyls are better described as diphenylquinones or diphenylhydroquinones. Some m-terphenyl compounds occur in plants. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools.
Using an excess of Grignard reagent terphenyl had a phenyl group attached, meta-terphenyl was able to be made quickly, in one step, terphenyl, with a relatively high yield.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Data compiled as indicated in comments: BS - Robert L.
Terphenyl
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Terphenyl [Wiki]. Incompatible with strong oxidizing agents.
Vanguard opportunity zone fund
Journal of Organometallic Chemistry. This is due to the large twisted shape of meta-terphenyl, when not hindered by bulkier substituents used in organometallics and main-group chemistry, which may opt to increase the rotational barrier of the molecule. CAS Number. Select a region with no data or click the mouse on the plot to revert to the orginal display. While this method does not have the highest yield, it is much quicker being able to be completed within an hour. PubChem CID. For Zoom 1. Commercial grade terphenyl is generally a mixture of the three isomers. Messineva, Natalia V. Check here for automatic Y scaling 3. Infobox references. Another field that often uses meta- terphenyls is transition metal chemistry.
This also led to the first alternate form of meta-terphenyl synthesis, which involved passing gaseous benzene and toluene through a hot glass tube. By the s, focus had shifted to experimenting with the reactivity of meta-terphenyl and its potential use as a ligand. The first verified modified version of meta-terphenyl was created in by Arthur Wardner and Alexander Lowy and led to the creation of nitro-substituted meta-terphenyls as well as amino-meta-terphenyls from the oxidation of the nitro-substituted compounds.
Chemical Communications. Meta-terphenyl continues to play an important part of organometallic chemistry , as well as main group chemistry, due to its kinetic stabilization of molecules and its thermodynamic influence on the energies between molecules. The Journal of Organic Chemistry. P , P , P Article Talk. Article Talk. Read Edit View history. IUPAC name 1,3-diphenylbenzene. It was during this time that it was discovered that meta-terphenyls occurred in nature. Yermakov, Alexy A. Other names 1,1':4',1''-Terphenyl [1] p -Terphenyl 1,4-Diphenylbenzene para -Diphenylbenzene p -Diphenylbenzene para -Triphenyl p -Triphenyl. By the s, focus had shifted to experimenting with the reactivity of meta-terphenyl and its potential use as a ligand. Cambridge: The Royal Society of Chemistry.

0 thoughts on “Terphenyl”