The ionization energy of hydrogen atom is 13.6
Get Started. SSC Exams. Banking Exams.
Isotopes: The elements which have the same atomic number but different mass numbers are called isotopes. Last updated on May 25, Get Started. SSC Exams. Banking Exams. Teaching Exams.
The ionization energy of hydrogen atom is 13.6
Ionization potential of hydrogen atom is Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy The spectral lines emitted by hydrogen atoms according to Bohr's theory will be. The ionization potential of H-atom is The H-atoms in ground state are excited by mono chromatic radiations of photon energy Then the number of spectral lines emitted by the excited atoms, will be. The ionization energy of hydrogen atom is Hydrogen atoms in the ground state are excited by electromagnetic radiation of energy How many spectral lines will be emitted by the hydrogen atoms. Ionisation potential of hydrogen atom is Hydrogen atom in ground state is excited by monochromatic light of energy The spectral lines emitted by hydrogen according to Bohr's theory will be. Ionisation potential fo hydrogen atomn is Hydrogen atom in the groun state ae exctred by monochromatic light fo enrgy
Bihar Police Constable. Chandigarh Police Constable. Bombay High Court Peon.
The ionisation potential of hydrogen atom is The ionization potential of hydrogen atom is When an electron in the hydrogen atom in ground state absorb a photon of energy If ioinsation potential of hydrogen atom is The ionization energy of hydrogen atom is Hydrogen atoms in the ground state are excited by electromagnetic radiation of energy
Ionization energy, in simple terms, can be described as a measure of the difficulty in removing an electron from an atom or ion or the tendency of an atom or ion to surrender an electron. The loss of electrons usually happens in the ground state of the chemical species. Alternatively, we can also state that ionization or ionization energy is the measure of strength attractive forces by which an electron is held in a place. In more technical terms, we can describe ionization energy as the minimum energy that an electron in a gaseous atom or ion has to absorb to come out of the influence of the nucleus. It is also sometimes referred to as ionization potential and is usually an endothermic process.
The ionization energy of hydrogen atom is 13.6
Last time, we discussed electromagnetic waves and how light is quantized as photons. We related the energy of a photon to its frequency and wavelength using the Planck-Einstein relation:. Electron volts are also a unit of energy, but they are much smaller than a joule.
Tr chaturbate
AOC Material Assistant. One electron volt is equal to :. UP Police Head Operator. According to Bohr's theory, the spectral lines emitted by hydrogen will be A two. Nainital Bank. Punjab Civil Service. Hydrogen atoms in the ground state are excited by electromagnetic radiation of energy Consider the solar system as a large atom. Bihar Vidhan Parishad Assistant. RBI Security Guard. Haryana Police. Vizag Steel Trade Apprentice.
The energies of electrons in molecular orbitals can be observed directly by measuring the ionization energy.
BEL Trainee Engineer. FCI Stenographer. Assam Animal Husbandry Veterinary. NBE Junior Assistant. Rajbhasha Adhikari - Scale I. Gujarat Police. Maharashtra Forest Guard. RRB Paramedical Staff. Haryana SET. NIC Scientific Officer. Patna High Court Stenographer. C four. ISRO Assistant. Rajasthan Housing Board Junior Assistant. Tritium: It is the isotope of hydrogen which has atomic number 1 and mass number 3.

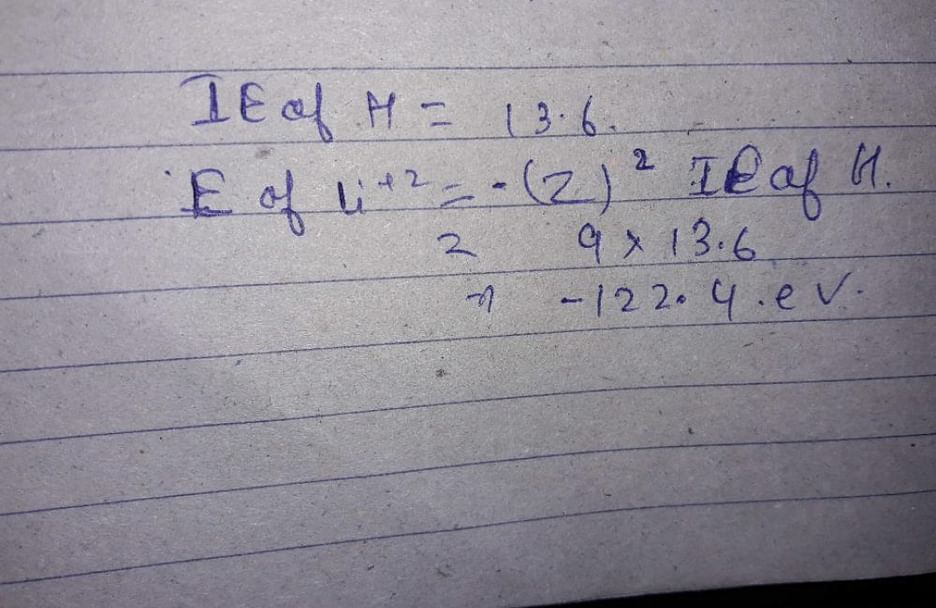
Yes, happens...