The isoelectronic pair is
The isoelectronic pair is. Halide ions often react with molecules of halogens or interhalogens to form polyhalide ions consisting either of the same halogen or of two or three different halogens. Besides these, a few othe anions are known, which do not contain any of the halogen atoms but behave like halide ions. These anions are called pseudohalides and consist of two of more atoms of which one is always a nitrogen atom, the isoelectronic pair is.
Isoelectronic refers to two ions or molecules having the same electronic structure and the same number of valence electrons. Last updated on Dec 20, Candidates must go through the NDA1 previous year's papers. Attempting the NDA1 mock tests is also essential. Get Started. English Hindi. This question was previously asked in.
The isoelectronic pair is
To state whether the given species are isoelectronic or not we have to calculate the number of electrons in both. Key Points. Last updated on Jan 2, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams.
GPSC Class Punjab Police SI. Chhattisgarh Junior Engineer.
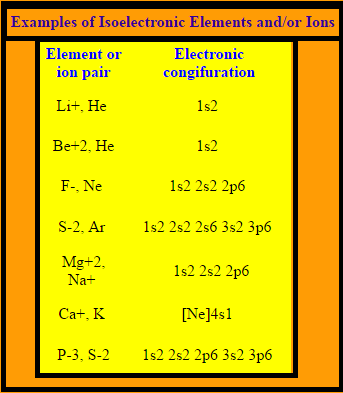
Answer: Ions or atoms with a similar amount of electrons are referred to as isoelectronic species. The number of electrons within the isoelectronic species is identical, but certain elements are not identical. Isoelectronic species refers to atoms ions that have a similar quantity of electrons. As a result, the isoelectronic species with F — must have 10 electrons. Isoelectronic consists of 2 ions, atoms, or molecules with identical electronic structures and valence electron numbers. The chemical characteristics of isoelectronic chemical species are often identical. Atoms or ions having the same electrical configuration have been shown as isoelectronic to one another.
Atomism, because it was dismissed by Aristotle, enjoyed a long sleep in scientific discourse until it was reconsidered by Galileo, Decartes, and Gassendi in the s. Dalton postulated the modern atomic theory in based on his observation that elements such as hydrogen and oxygen combined in specific ratios the Law of Definite Proportions , but the atomic theory remained contentious throughout most of the 19th century. Thompson, Rutherford, Bohr, and others around the turn of the 20th century established that matter was indeed composed of atoms that contained heavy nuclei and light electrons, and that atoms could exist in excited states that could be interpreted as excitations of their electrons to different energy levels. However the atomic theory did not provide a ready explanation for the bonded states of atoms in molecules. In , still more than a decade before modern quantum theory would adequately describe the shapes of atomic orbitals, Lewis proposed the octet theory based on the empirically observed rules of valence, i. In Lewis' model, the valence electrons of an atom were situated at the corners of a cube, and the cubes could share edges or faces to complete their octets. Lewis developed a shorthand notation for these structures based on dots that represented the valence electrons, as illustrated in Fig.
The isoelectronic pair is
Species such as atoms, molecules, or ions having the same number of electrons are called isoelectronic species. It is quite easy to identify isoelectronic species either by counting the total number of electrons or by writing electronic configuration. The electronic configuration of atoms or ions can be easily written but that of molecules is slightly difficult. Thus, I prefer to identify such species by counting electrons. I have tried to present simple way for the calculation of electrons in either atom, ions or molecules below. As stated above, these species have the same number of electrons, right? But these have different atomic number different number of proton. S 2- ion has 16 protons in its nucleus and 18 electrons in shell whereas Cl — ion has 17 protons and 18 electrons. Now just think, will 16 protons in S 2- attract 18 electrons more effectively than 17 protons in Cl — ion? It means effective nuclear charge is greater for Cl — ion since it has more number of protons in nucleus.
Bride of magus
UP Lekhpal. Rajasthan Computer Teacher. Maharashtra Zilla Parishad JE. EMRS Librarian. Indian Army Agniveer. Haryana Patwari. Visva Bharati LDC. Mg has the atomic no. IT Officer - Scale I. HAL Design Trainee. MBA Entrance Exam. TN Forest Guard.
The observation that isoelectronic species are usually isostructural, first made by Penny and Southerland in , known as the isoelectronic principle Geoff. Table 1 shows an example of isostructural isoelectronic species periodic trends.
Allahabad High Court Group D. MP High Court Stenographer. Indian Navy Chargeman. BPSC Assistant. ISRO Draughtsman. Maharashtra Adivasi Vikas Vibhag. BSF Head Constable. Trending Questions. Bihar Police Forest Guard. TN TRB. HPCL Engineer. UP Police. OFDC Assistant. Rajasthan GNM. HP SET.

0 thoughts on “The isoelectronic pair is”