The outer electronic configuration of gd
The lanthanoid follow the 4f 5d 6s 2 configuration common configuration with some exception due to full filled half filled electronic configuration. JEE Main session 2 registration ends tomorrow; options to login, image instructions.
Doc 25 Pages. Sign in Open App. The outer electronic configuration of Gd Atomic number 64 is. Verified Answer. All the electrons in orbital are unpaired, hence stable. Related Content.
The outer electronic configuration of gd
This action cannot be undone. This will permanently delete All Practiced Questions. In the long form of periodic table, the elements having lowest ionization potential are placed in:. Elements with an electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 3 belong to the group :. Which one of the elements with the following outer orbital configurations may exhibit the largest number of oxidation states? Are you sure? Clear Question Attempted. Botany All. Chemistry All. Physics All. Units and Measurement All Select Topic.
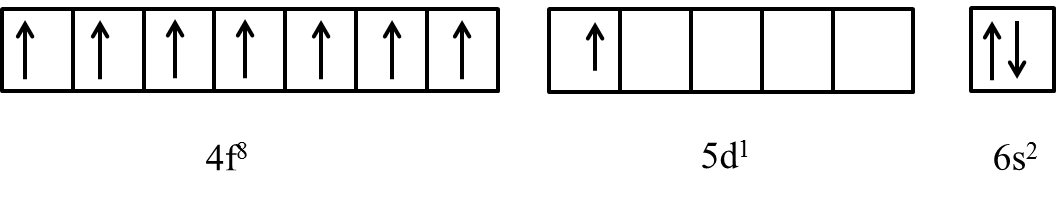
Besides giving tempstars explanation of The outer electronic configuration of Gd Atomic number 64 is AIEEE a 4f3, 5d5, 6s2b 4f8,d0, 6s2c 4f4,5d4,6s2d 4f7, 5d1,6s2Correct answer is option 'D'. Low m.
Ionisation potential of hydrogen atom is Hydrogen atom is ground state is excited by monochromatic light of energy The spectral lines emitted by hydrogen according to bohr's theory will be-. Which one of the following is associated with a de Broglie wave of longer wavelength-a proton or an electron moving with same velocity? Maximum deviation from ideal gas is expected in case of-. The outer electronic configuration of Gd At.
Gadolinium is a classified lanthanide element. In this article, I have discussed in detail how to easily write the complete electron configuration of gadolinium. The total number of electrons in gadolinium is sixty-four. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in gadolinium in specific rules in different orbits and orbitals is called the electron configuration of gadolinium. The electron configuration of gadolinium is [ Xe ] 4f 7 5d 1 6s 2 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.
The outer electronic configuration of gd
The commonly used long form of the periodic table is designed to emphasize electron configurations. Since it is the outermost valence electrons which are primarily involved in chemical interactions between atoms, the last electron added to an atom in the building-up process is of far more interest to a chemist than the first. This last electron is called the distinguishing electron because it distinguishes an atom from the one immediately preceding it in the periodic table. The type of subshell s, p, d, f into which the distinguishing electron is placed is very closely related to the chemical behavior of an element and gives rise to the classification shown by the color-coding on the periodic table seen here. The representative elements are those in which the distinguishing electron enter an s or p subshell.
Katherine heigl nude pics
Text Solution. Create Your Account Name. View all answers and join this discussion on the EduRev App. Gender Male Female Others. School Change. Forgot Password. Units and Measurement All Select Topic. Total number of orbitals in 4th energy level of an atom is- Chemistry All. View more. Com Colleges in Mumbai Top B. Top Courses for JEE. Video Solution. View all.
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells.
Which of the following is the energy of a possible excited state of hy View answers on App. Subtopic: Evolution of Periodic Table. Create Your Account Name. Inversely proportional to the effective nuclear charge. Signup to see your scores go up within 7 days! Posted by prateek. Directly proportional to the effective nuclear charge. Doc 25 Pages. Select a course to view your unattempted tests.

I join. I agree with told all above. We can communicate on this theme.